|
Dimethyldithiocarbamate
Dimethyldithiocarbamate is the organosulfur anion with the formula (CH3)2NCS2−. It is one of the simplest organic dithiocarbamate. Uses It is a component of various pesticides and rubber chemicals in the form of its salts sodium dimethyldithiocarbamate, and potassium dimethyldithiocarbamate) as well as its complexes zinc dimethyldithiocarbamate, ferric dimethyldithiocarbamate, and nickel bis(dimethyldithiocarbamate). Oxidation gives thiram Thiram is the simplest thiuram disulfide and the oxidized dimer of dimethyldithiocarbamate. It is used as a fungicide, ectoparasiticide to prevent fungal diseases in seed and crops and similarly as an animal repellent to protect fruit trees and o .... 222px, Iron tris(dimethyldithiocarbamate) (Fe(S2CNMe2)3) is illustrative of hundreds of known dithiocarbamate complexes. References Dithiocarbamates Pesticides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
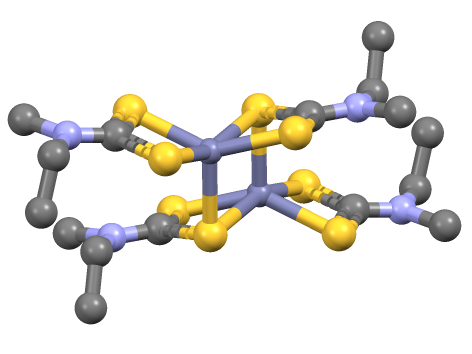
Ferric Dimethyldithiocarbamate
Iron tris(dimethyldithiocarbamate) is the coordination complex of iron with dimethyldithiocarbamate with the formula Fe(S2CNMe2)3 (Me = methyl). It is marketed as a fungicide. Synthesis, structure, bonding Iron tris(dithiocarbamate)s are typically are prepared by salt metathesis reactions. Iron tris(dimethyldithiocarbamate) is an octahedral coordination complex of iron(III) with D3 symmetry. Spin crossover (SCO) was first observed in 1931 by Cambi ''et al.'' who discovered anomalous magnetic behavior for the tris(N,N-dialkyldithiocarbamatoiron(III) complexes. The spin states of these complexes are sensitive to the nature of the amine substituents. Iron tris(dithiocarbamate)s characteristically react with nitric oxide to give Fe(dtc)2NO. This efficient chemical trapping reaction provides a means to detect NO. Reflecting the strongly donating properties of dithiocarbamate ligands, iron tris(dithiocarbamate)s oxidize at relatively mild potentials to give isolable iron(IV) deri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Dimethyldithiocarbamate
Zinc dimethyldithiocarbamate is a coordination complex of zinc with dimethyldithiocarbamate. It is a pale yellow solid that is used as a fungicide, the sulfur vulcanization of rubber, and other industrial applications. Applications Known as ziram in agriculture, it was introduced in the United States in 1960 as a broad-spectrum fungicide. It was used to address scab on apples and pears, leaf curl in peaches, and anthracnose and blight in tomatoes. In 1981, additional uses for ziram were approved, including the prevention of leaf blight and scab on almonds, shot-hole in apricots, brown rot and leaf spot in cherries, and scab and anthracnose in pecans. Ziram also began to be used on residential ornaments as a bird and mammal repellent. As a protectant fungicide, it active on the plant’s surface where it forms a chemical barrier between the plant and a fungus. A protectant fungicide is not absorbed into the plant and must be applied prior to infection. Ziram can either be direct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyldithiocarbamate
Dimethyldithiocarbamate is the organosulfur anion with the formula (CH3)2NCS2−. It is one of the simplest organic dithiocarbamate. Uses It is a component of various pesticides and rubber chemicals in the form of its salts sodium dimethyldithiocarbamate, and potassium dimethyldithiocarbamate) as well as its complexes zinc dimethyldithiocarbamate, ferric dimethyldithiocarbamate, and nickel bis(dimethyldithiocarbamate). Oxidation gives thiram Thiram is the simplest thiuram disulfide and the oxidized dimer of dimethyldithiocarbamate. It is used as a fungicide, ectoparasiticide to prevent fungal diseases in seed and crops and similarly as an animal repellent to protect fruit trees and o .... 222px, Iron tris(dimethyldithiocarbamate) (Fe(S2CNMe2)3) is illustrative of hundreds of known dithiocarbamate complexes. References Dithiocarbamates Pesticides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel Bis(dimethyldithiocarbamate)
Nickel bis(dimethyldithiocarbamate) is the coordination complex on nickel and dimethyldithiocarbamate, with the formula Ni(S2CNMe2)2 (Me = methyl). It is the prototype for a large number of bis(dialkhyldithiocarbamate)s of nickel(II), which feature diverse organic substituents, all of which have similar structures. Nickel bis(dimethyldithiocarbamate) has been marketed as a fungicide and related complexes are used as stabilizers in polymers. Preparation and structure The compound precipitates as a black solid upon combining aqueous solutions of salts of nickel(II) and dimethyldithiocarbamate. In terms of structure and bonding the nickel is square planar, and the complex is diamagnetic.{{cite journal, title=The Chemistry of the Dithioacid and 1,1-Dithiolate Complexes, journal=Progress in Inorganic Chemistry, volume=11, author=D. Coucouvanis, pages=233–371, doi=10.1002/9780470166123.ch4 See also * Zinc dimethyldithiocarbamate - a related compound where nickel has been replaced w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiocarbamate
In organic chemistry, a dithiocarbamate is a functional group with the general formula and structure . It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms (when only 1 oxygen is replaced the result is thiocarbamate). A common example is sodium diethyldithiocarbamate. Dithiocarbamates and their derivatives are widely used in the vulcanization of rubber. Formation Many primary and secondary amines react with carbon disulfide and sodium hydroxide to form dithiocarbamate salts: :R2NH + CS2 + NaOH → R2NCS2−Na+ + H2O Ammonia reacts with CS2 similarly: :2 NH3 + CS2 → H2NCS2−NH4+ Dithiocarbamate salts are pale colored solids that are soluble in water and polar organic solvents. Reactions Dithiocarbamates are readily S-alkylated. Thus, methyl dimethyldithiocarbamate can be prepared by methylation of the dithiocarbamate: :(CH3)2NCS2Na + (CH3O)2SO2 → (CH3)2NC(S)SCH3 + Na H3OSO3 Oxidation of dithiocarbamates gives th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiocarbamates
In organic chemistry, a dithiocarbamate is a functional group with the general formula and structure . It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms (when only 1 oxygen is replaced the result is thiocarbamate). A common example is sodium diethyldithiocarbamate. Dithiocarbamates and their derivatives are widely used in the vulcanization of rubber. Formation Many primary and secondary amines react with carbon disulfide and sodium hydroxide to form dithiocarbamate salts: :R2NH + CS2 + NaOH → R2NCS2−Na+ + H2O Ammonia reacts with CS2 similarly: :2 NH3 + CS2 → H2NCS2−NH4+ Dithiocarbamate salts are pale colored solids that are soluble in water and polar organic solvents. Reactions Dithiocarbamates are readily S-alkylated. Thus, methyl dimethyldithiocarbamate can be prepared by methylation of the dithiocarbamate: :(CH3)2NCS2Na + (CH3O)2SO2 → (CH3)2NC(S)SCH3 + Na H3OSO3 Oxidation of dithiocarbamates gives the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiram
Thiram is the simplest thiuram disulfide and the oxidized dimer of dimethyldithiocarbamate. It is used as a fungicide, ectoparasiticide An ectoparasiticide is an antiparasitic drug used in the treatment of ectoparasitic infestations. These drugs are used to kill the parasites that live on the body surface. Permethrin, sulfur, lindane, dicophane, benzyl benzoate, ivermectin and crot ... to prevent fungal diseases in seed and crops and similarly as an animal repellent to protect fruit trees and ornamentals from damage by rabbits, rodents and deer. It is effective against Stem gall of coriander, damping off, smut of millet, neck rot of onion, etc. Thiram has been used in the treatment of human scabies, as a sun screen and as a bactericide applied directly to the skin or incorporated into soap. Thiram is also used as a sulfur source and secondary accelerator the sulfur vulcanization of rubbers. Usage Thiram was traditionally used in apple and wine farming. Since 2010 most thiram ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosulfur Compound
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two ( cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries. Sulfur shares the chalcogen group with oxygen, selenium, and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium, and carbon–tellurium compounds. A classical chemical test for the detection of sulfur co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pesticide
Pesticides are substances that are meant to control pests. This includes herbicide, insecticide, nematicide, molluscicide, piscicide, avicide, rodenticide, bactericide, insect repellent, animal repellent, microbicide, fungicide, and lampricide. The most common of these are herbicides which account for approximately 80% of all pesticide use. Most pesticides are intended to serve as plant protection products (also known as crop protection products), which in general, protect plants from weeds, fungi, or insects. As an example, the fungus ''Alternaria solani'' is used to combat the aquatic weed ''Salvinia''. In general, a pesticide is a chemical (such as carbamate) or biological agent (such as a virus, bacterium, or fungus) that deters, incapacitates, kills, or otherwise discourages pests. Target pests can include insects, plant pathogens, weeds, molluscs, birds, mammals, fish, nematodes (roundworms), and microbes that destroy property, cause nuisance, or spread disease, or a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions. The component ions in a salt compound can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as fluoride (F−), or polyatomic, such as sulfate (). Types of salt Salts can be classified in a variety of ways. Salts that produce hydroxide ions when dissolved in water are called ''alkali salts'' and salts that produce hydrogen ions when dissolved in water are called ''acid salts''. ''Neutral salts'' are those salts that are neither acidic nor basic. Zwitterions contain an anionic and a cationic centre in the same molecule, but are not considered salts. Examples of zwitterions are amino acids, many metabolites, peptid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Environmental Protection Agency
A biophysical environment is a biotic and abiotic surrounding of an organism or population, and consequently includes the factors that have an influence in their survival, development, and evolution. A biophysical environment can vary in scale from microscopic to global in extent. It can also be subdivided according to its attributes. Examples include the marine environment, the atmospheric environment and the terrestrial environment. The number of biophysical environments is countless, given that each living organism has its own environment. The term ''environment'' can refer to a singular global environment in relation to humanity, or a local biophysical environment, e.g. the UK's Environment Agency. Life-environment interaction All life that has survived must have adapted to the conditions of its environment. Temperature, light, humidity, soil nutrients, etc., all influence the species within an environment. However, life in turn modifies, in various forms, its conditions. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |