|
Diels–Alder Reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally-allowed +2cycloaddition with Woodward–Hoffmann symbol π4s_+_π2s.html" ;"title="sub>π4s + π2s">sub>π4s + π2s It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quinol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereospecific
In chemistry, stereospecificity is the property of a reaction mechanism that leads to different stereoisomeric reaction products from different stereoisomeric reactants, or which operates on only one (or a subset) of the stereoisomers."Overlap Control of Carbanionoid Reactions. I. Stereoselectivity in Alkaline Epoxidation," Zimmerman, H. E.; Singer, L.; Thyagarajan, B. S. J. Am. Chem. Soc., 1959, 81, 108-116.Eliel, E., "Stereochemistry of Carbon Compound", McGraw-Hill, 1962 pp 434-436 In contrast, stereoselectivity is the property of a reactant mixture where a non-stereospecific mechanism allows for the formation of multiple products, but where one (or a subset) of the products is favored by factors, such as steric access, that are independent of the mechanism. A stereospecific mechanism ''specifies'' the stereochemical outcome of a given reactant, whereas a stereoselective reaction ''selects'' products from those made available by the same, non-specific mechanism acting on a giv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
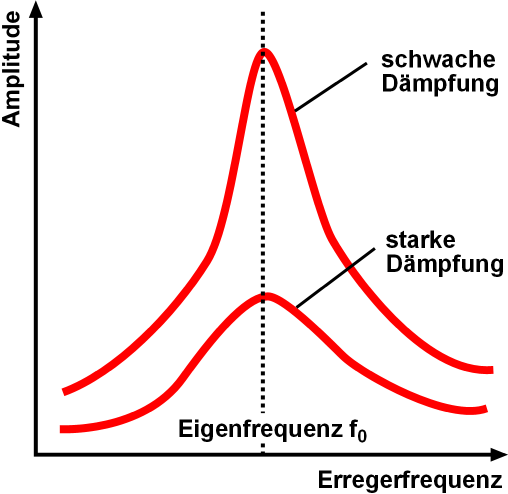
Resonance Of Diene And Dienophile
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied Periodic function, periodic force (or a Fourier analysis, Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an Oscillation, oscillating force is applied at a resonant frequency of a dynamic system, the system will oscillate at a higher Amplitude, amplitude than when the same force is applied at other, non-resonant frequencies. Frequencies at which the response amplitude is a relative maximum are also known as resonant frequencies or resonance frequencies of the system. Small periodic forces that are near a resonant frequency of the system have the ability to produce large amplitude oscillations in the system due to the storage of vibrational energy. Resonance phenomena occur with all types of vibrations or waves: there is mechanical resonance, orbital resonance, acoustic resonance, Electromagnetic radiation, electromagnet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition State
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked with the double dagger ‡ symbol. As an example, the transition state shown below occurs during the SN2 reaction of bromoethane with a hydroxide anion: The activated complex of a reaction can refer to either the transition state or to other states along the reaction coordinate between reactants and products, especially those close to the transition state.Peter Atkins and Julio de Paula, ''Physical Chemistry'' (8th ed., W.H. Freeman 2006), p.809 According to the transition state theory, once the reactants have passed through the transition state configuration, they always continue to form products. History of concept The concept of a transition state has been important in many theories of the rates at which chemical reactions occ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intramolecular Diels–Alder Cycloaddition
In organic chemistry, an intramolecular Diels-Alder cycloaddition is a Diels–Alder reaction in which the diene and a dienophile are both part of the same molecule. The reaction leads to the formation of the same cyclohexene-like structure as usual for a Diels–Alder reaction, but as part of a more complex fused or bridged cyclic ring system. This reaction gives rise to various natural derivatives of decalin. Reaction products Because the two reacting groups are already attached, two basic modes of addition are possible in this reaction. Depending on whether the tether that links to the dienophile is attached to the end or the middle of the diene, fused or bridged polycyclic ring systems can be formed. The tether than attaches the two reacting groups also affects the geometry of the reaction. As a result of its conformational and other structural restrictions, the exo vs endo results are usually not based on the simple (intermolecular) Diels–Alder reaction effects. Applica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Constitutional Isomer
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a chemical compound, compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct chemical bond, bonds between them. The term metamer was formerly used for the same concept. For example, butanol , methyl propyl ether , and diethyl ether have the same molecular formula but are three distinct structural isomers. The concept applies also to polyatomic ions with the same total charge. A classical example is the cyanate ion and the fulminate ion . It is also extended to ionic compounds, so that (for example) ammonium cyanate and urea are considered structural isomers,William F. Bynum, E. Janet Browne, Roy Porter (2014): ''Dictionary of the History of Science''. 530 pages. and so are methylammonium formate and ammonium acetate . Structural isomerism is the most radical type of isomerism. It is opposed to stereoisomerism, in which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermolecular
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction or repulsion which act between atoms and other types of neighbouring particles, e.g. atoms or ions. Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics. The investigation of intermolecular forces starts from macroscopic observations which indicate the existence and action of forces at a molecular level. These observations include non-ideal-gas thermodynamic behavior reflected by virial coefficients, vapor pressure, viscosity, superficial tension, and absorption data. The first reference to the nature of microscopic forc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FMO Of Diels-Alder Reaction (Dutch: ')
{{disambiguation ...
FMO may refer to: * Fenna-Matthews-Olson complex * Fish Marketing Organisation, a statutory body of Hong Kong * Flavin-containing monooxygenase * Flexible macroblock ordering * Fragment molecular orbital * Frontier molecular orbital theory * Münster Osnabrück International Airport in North Rhine-Westphalia, Germany * Netherlands Development Finance Company FMO ( nl, Nederlandse Financierings-Maatschappij voor Ontwikkelingslanden N.V.) is a Dutch development bank structured as a bilateral private-sector international financial institution based in the Hague, the Netherlands. FMO manages funds for the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inverse Electron-demand Diels–Alder Reaction
The inverse electron demand Diels–Alder reaction, or DAINV or IEDDA is an organic chemical reaction, in which two new chemical bonds and a six-membered ring are formed. It is related to the Diels–Alder reaction, but unlike the Diels–Alder (or DA) reaction, the DAINV is a cycloaddition between an electron-rich dienophile and an electron-poor diene. During a DAINV reaction, three pi-bonds are broken, and two sigma bonds and one new pi-bond are formed. A prototypical DAINV reaction is shown on the right. DAINV reactions often involve heteroatoms, and can be used to form heterocyclic compounds. This makes the DAINV reaction particularly useful in natural product syntheses, where the target compounds often contain heterocycles. Recently, the DAINV reaction has been used to synthesize a drug transport system which targets prostate cancer. History The Diels–Alder reaction was first reported in 1928 by Otto Diels and Kurt Alder; they were awarded the Nobel Prize in chemistr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frontier Molecular Orbital Theory
In chemistry, frontier molecular orbital theory is an application of MO theory describing HOMO/LUMO interactions. History In 1952, Kenichi Fukui published a paper in the ''Journal of Chemical Physics'' titled "A molecular theory of reactivity in aromatic hydrocarbons." Though widely criticized at the time, he later shared the Nobel Prize in Chemistry with Roald Hoffmann for his work on reaction mechanisms. Hoffman's work focused on creating a set of four pericyclic reactions in organic chemistry, based on orbital symmetry, which he coauthored with Robert Burns Woodward, entitled "The Conservation of Orbital Symmetry." Fukui's own work looked at the frontier orbitals, and in particular the effects of the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO) on reaction mechanisms, which led to it being called Frontier Molecular Orbital Theory (FMO Theory). He used these interactions to better understand the conclusions of the Woodward–Hoffma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |