|
Dideoxynucleotides
Dideoxynucleotides are chain-elongating inhibitors of DNA polymerase, used in the Sanger method for DNA sequencing. They are also known as 2',3' because both the 2' and 3' positions on the ribose lack hydroxyl groups, and are abbreviated as ''ddNTPs'' (ddGTP, ddATP, ddTTP and ddCTP). Role in the Sanger method The Sanger method is used to amplify a target segment of DNA, so that the DNA sequence can be determined precisely. The incorporation of ddNTPs in the reaction valves are simply used to terminate the synthesis of a growing DNA strand, resulting in partially replicated DNA fragments. This is because DNA polymerase requires the 3' OH group of the growing chain and the 5' phosphate group of the incoming dNTP to create a phosphodiester bond. Sometimes the DNA polymerase will incorporate a ddNTP and the absence of the 3' OH group will interrupt the condensation reaction between the 5' phosphate (following the cleavage of pyrophospate) of the incoming nucleotide with the 3' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DNA Sequencing
DNA sequencing is the process of determining the nucleic acid sequence – the order of nucleotides in DNA. It includes any method or technology that is used to determine the order of the four bases: adenine, guanine, cytosine, and thymine. The advent of rapid DNA sequencing methods has greatly accelerated biological and medical research and discovery. Knowledge of DNA sequences has become indispensable for basic biological research, DNA Genographic Projects and in numerous applied fields such as medical diagnosis, biotechnology, forensic biology, virology and biological systematics. Comparing healthy and mutated DNA sequences can diagnose different diseases including various cancers, characterize antibody repertoire, and can be used to guide patient treatment. Having a quick way to sequence DNA allows for faster and more individualized medical care to be administered, and for more organisms to be identified and cataloged. The rapid speed of sequencing attained with modern D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dideoxy Termination Of DNA Elongation EN
Dideoxynucleotides are chain-elongating inhibitors of DNA polymerase, used in the Sanger method for DNA sequencing. They are also known as 2',3' because both the 2' and 3' positions on the ribose lack hydroxyl groups, and are abbreviated as ''ddNTPs'' (ddGTP, ddATP, ddTTP and ddCTP). Role in the Sanger method The Sanger method is used to amplify a target segment of DNA, so that the DNA sequence can be determined precisely. The incorporation of ddNTPs in the reaction valves are simply used to terminate the synthesis of a growing DNA strand, resulting in partially replicated DNA fragments. This is because DNA polymerase requires the 3' OH group of the growing chain and the 5' phosphate group of the incoming dNTP to create a phosphodiester bond. Sometimes the DNA polymerase will incorporate a ddNTP and the absence of the 3' OH group will interrupt the condensation reaction between the 5' phosphate (following the cleavage of pyrophospate) of the incoming nucleotide with the 3' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
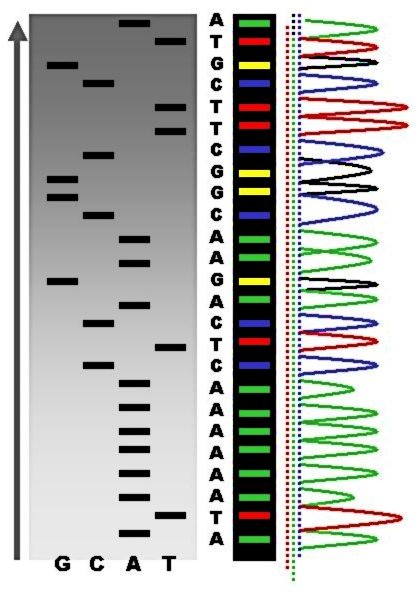
Sanger Sequencing
Sanger sequencing is a method of DNA sequencing that involves electrophoresis and is based on the random incorporation of chain-terminating dideoxynucleotides by DNA polymerase during in vitro DNA replication. After first being developed by Frederick Sanger and colleagues in 1977, it became the most widely used sequencing method for approximately 40 years. It was first commercialized by Applied Biosystems in 1986. More recently, higher volume Sanger sequencing has been replaced by next generation sequencing methods, especially for large-scale, automated genome analyses. However, the Sanger method remains in wide use for smaller-scale projects and for validation of deep sequencing results. It still has the advantage over short-read sequencing technologies (like Illumina) in that it can produce DNA sequence reads of > 500 nucleotides and maintains a very low error rate with accuracies around 99.99%. Sanger sequencing is still actively being used in efforts for public health initiative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chain Termination Method
Sanger sequencing is a method of DNA sequencing that involves electrophoresis and is based on the random incorporation of chain-terminating dideoxynucleotides by DNA polymerase during in vitro DNA replication. After first being developed by Frederick Sanger and colleagues in 1977, it became the most widely used sequencing method for approximately 40 years. It was first commercialized by Applied Biosystems in 1986. More recently, higher volume Sanger sequencing has been replaced by next generation sequencing methods, especially for large-scale, automated genome analyses. However, the Sanger method remains in wide use for smaller-scale projects and for validation of deep sequencing results. It still has the advantage over short-read sequencing technologies (like Illumina) in that it can produce DNA sequence reads of > 500 nucleotides and maintains a very low error rate with accuracies around 99.99%. Sanger sequencing is still actively being used in efforts for public health initiat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uridine
Uridine (symbol U or Urd) is a glycosylated pyrimidine analog containing uracil attached to a ribose ring (or more specifically, a ribofuranose) via a β-N1-glycosidic bond. The analog is one of the five standard nucleosides which make up nucleic acids, the others being adenosine, thymidine, cytidine and guanosine. The five nucleosides are commonly abbreviated to their symbols, U, A, dT, C, and G, respectively. However, thymidine is more commonly written as 'dT' ('d' represents 'deoxy') as it contains a 2'-deoxyribofuranose moiety rather than the ribofuranose ring found in uridine. This is because thymidine is found in deoxyribonucleic acid (DNA) and usually not in ribonucleic acid (RNA). Conversely, uridine is found in RNA and not DNA. The remaining three nucleosides may be found in both RNA and DNA. In RNA, they would be represented as A, C and G whereas in DNA they would be represented as dA, dC and dG. Biosynthesis Uridine is widely produced in nature as uridine monophosp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deoxynucleotides
A deoxyribonucleotide is a nucleotide that contains deoxyribose. They are the monomeric units of the informational biopolymer, deoxyribonucleic acid ( DNA). Each deoxyribonucleotide comprises three parts: a deoxyribose sugar ( monosaccharide), a nitrogenous base, and one phosphoryl group. The nitrogenous bases are either purines or pyrimidines, heterocycles whose structures support the specific base-pairing interactions that allow nucleic acids to carry information. The base is always bonded to the 1'-carbon of the deoxyribose, an analog of ribose in which the hydroxyl group of the 2'-carbon is replaced with a hydrogen atom. The third component, the phosphoryl group, attaches to the deoxyribose monomer via the hydroxyl group on the 5'-carbon of the sugar. When deoxyribonucleotides polymerize to form DNA, the phosphate group from one nucleotide will bond to the 3' carbon on another nucleotide, forming a phosphodiester bond via dehydration synthesis. New nucleotides are always add ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Taq Polymerase
''Taq'' polymerase is a thermostable DNA polymerase I named after the thermophilic eubacterial microorganism ''Thermus aquaticus,'' from which it was originally isolated by Chien et al. in 1976. Its name is often abbreviated to ''Taq'' or ''Taq'' pol. It is frequently used in the polymerase chain reaction (PCR), a method for greatly amplifying the quantity of short segments of DNA. ''T. aquaticus'' is a bacterium that lives in hot springs and hydrothermal vents, and ''Taq'' polymerase was identified as an enzyme able to withstand the protein-denaturing conditions (high temperature) required during PCR. Therefore, it replaced the DNA polymerase from '' E. coli'' originally used in PCR. Enzymatic properties ''Taqs optimum temperature for activity is 75–80 °C, with a half-life of greater than 2 hours at 92.5 °C, 40 minutes at 95 °C and 9 minutes at 97.5 °C, and can replicate a 1000 base pair strand of DNA in less than 10 seconds at 72 °C. At ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complementary DNA
In genetics, complementary DNA (cDNA) is DNA synthesized from a single-stranded RNA (e.g., messenger RNA (mRNA) or microRNA (miRNA)) template in a reaction catalyzed by the enzyme reverse transcriptase. cDNA is often used to express a specific protein in a cell that does not normally express that protein (i.e., heterologous expression), or to sequence or quantify mRNA molecules using DNA based methods (qPCR, RNA-seq). cDNA that codes for a specific protein can be transferred to a recipient cell for expression, often bacterial or yeast expression systems. cDNA is also generated to analyze transcriptomic profiles in bulk tissue, single cells, or single nuclei in assays such as microarrays, qPCR, and RNA-seq. cDNA is also produced naturally by retroviruses (such as HIV-1, HIV-2, simian immunodeficiency virus, etc.) and then integrated into the host's genome, where it creates a provirus. The term ''cDNA'' is also used, typically in a bioinformatics context, to refer to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. Production About 200,000 tonnes of tetrahydrofuran are produced annually. The most widely used industrial process involves the acid-catalyzed dehydration of 1,4-butanediol. Ashland/ISP is one of the biggest producers of this chemical route. The method is similar to the production of diethyl ether from ethanol. The butanediol is derived from condensation of acetylene with formaldehyde followed by hydrogenation. DuPont developed a process for producing THF by oxidizing ''n''-butane to crude maleic anhydride, followed by catalytic hydrogenation. A third major industrial route entails hydroformylation of allyl alcohol followed by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleoside
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase (also termed a nitrogenous base) and a five-carbon sugar (ribose or 2'-deoxyribose) whereas a nucleotide is composed of a nucleobase, a five-carbon sugar, and one or more phosphate groups. In a nucleoside, the anomeric carbon is linked through a glycosidic bond to the N9 of a purine or the N1 of a pyrimidine. Nucleotides are the molecular building-blocks of DNA and RNA. List of nucleosides and corresponding nucleobases The reason for 2 symbols, shorter and longer, is that the shorter ones are better for contexts where explicit disambiguation is superfluous (because context disambiguates) and the longer ones are for contexts where explicit disambiguation is judged to be needed or wise. For example, when discussing long nucleobase sequences in genomes, the CATG symbol system is much preferable to the Cyt-Ade-Thy-Gua symbol system (see '' N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethyl Orthoformate
Trimethyl orthoformate (TMOF) is the organic compound with the formula HC(OCH3)3. A colorless liquid, it is the simplest orthoester. It is a reagent used in organic synthesis for the formation of methyl ethers. The product of reaction of an aldehyde with trimethyl orthoformate is an acetal. In general cases, these acetals can be deprotected back to the aldehyde by using hydrochloric acid. Synthesis Trimethyl orthoformate is prepared on an industrial scale by the methanolysis of hydrogen cyanide:Ashford's Dictionary of Industrial Chemicals, Third edition, 2011, , page 9388 :HCN + 3 HOCH3 → HC(OCH3)3 + NH3 Trimethyl orthoformate can also be prepared from the reaction between chloroform and sodium methoxide, an example of the Williamson ether synthesis. Use Trimethyl orthoformate is a useful building block for creating methoxymethylene groups and heterocyclic ring systems. It introduces a formyl group to a nucleophilic substrate, e.g. RNH2 to form R-NH-CHO, which can undergo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |