|
Desi Bouterse
{{disambig ...
DESI may refer to * Desorption electrospray ionization * Drug Efficacy Study Implementation * Dark Energy Spectroscopic Instrument See also * Desi (other) Desi or Deshi is a self-referential term used by South Asian people. Desi may also refer to: *Desi (raga), a raga (also known as Deshi) in Indian classical music * Desi daru, an Indian alcoholic beverage *Desi ghee, a term used to differentiate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desorption Electrospray Ionization
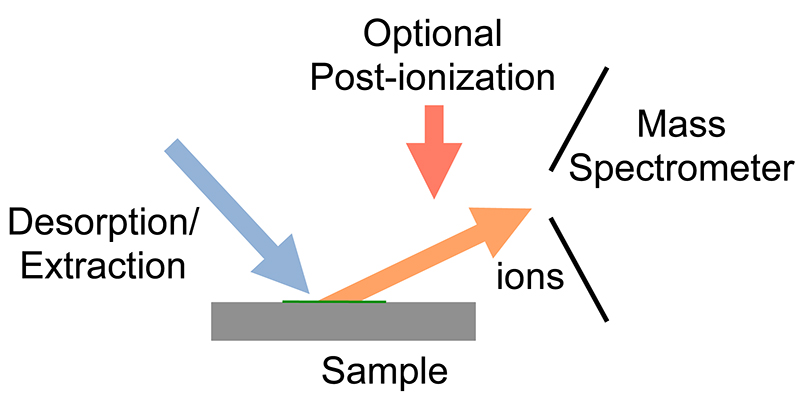
Desorption electrospray ionization (DESI) is an ambient ionization technique that can be coupled to mass spectrometry (MS) for chemical analysis of samples at atmospheric conditions. Coupled ionization sources-MS systems are popular in chemical analysis because the individual capabilities of various sources combined with different MS systems allow for chemical determinations of samples. DESI employs a fast-moving charged solvent stream, at an angle relative to the sample surface, to extract analytes from the surfaces and propel the secondary ions toward the mass analyzer. This tandem technique can be used to analyze forensics analyses, pharmaceuticals, plant tissues, fruits, intact biological tissues, enzyme-substrate complexes, metabolites and polymers. Therefore, DESI-MS may be applied in a wide variety of sectors including food and drug administration, pharmaceuticals, environmental monitoring, and biotechnology. History DESI has been widely studied since its inception in 2004 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Efficacy Study Implementation
Drug Efficacy Study Implementation (DESI) was a program begun by the Food and Drug Administration (FDA) in the 1960s after the requirement (in the Kefauver-Harris Drug Control Act) that all drugs be efficacious as well as safe, was made part of US law. The DESI program was intended to classify all pre-1962 drugs that were already on the market as either effective, ineffective, or needing further study.Pharmacy Today. August 2008.FDA aims to remove unapproved drugs from market: Risk-based enforcement program focuses on removing potentially harmful products/ref> The Drug Efficacy Study Implementation (DESI) evaluated over 3,000 separate products and over 16,000 therapeutic claims. By 1984, final action had been completed on 3,443 products; of these, 2,225 were found to be effective, 1,051 were found not effective, and 167 were pending. One of the early effects of the DESI study was the development of the Abbreviated New Drug Application (ANDA). See also * Estes Kefauver * Federa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dark Energy Spectroscopic Instrument
The Dark Energy Spectroscopic Instrument (DESI) is a scientific research instrument for conducting spectrographic astronomical surveys of distant galaxies. Its main components are a focal plane containing 5,000 fiber-positioning robots, and a bank of spectrographs which are fed by the fibers. The new instrument will enable an experiment to probe the expansion history of the universe and the mysterious physics of dark energy. The instrument is operated by the Lawrence Berkeley National Laboratory under funding from the US Department of Energy's Office of Science. Construction of the new instrument, now completed, was principally funded by the US Department of Energy's Office of Science, and by other numerous sources including the US National Science Foundation, the UK Science and Technology Facilities Council, France's Alternative Energies and Atomic Energy Commission, Mexico's National Council of Science and Technology, Spain's Ministry of Science and Innovation, by the Gordon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |