|
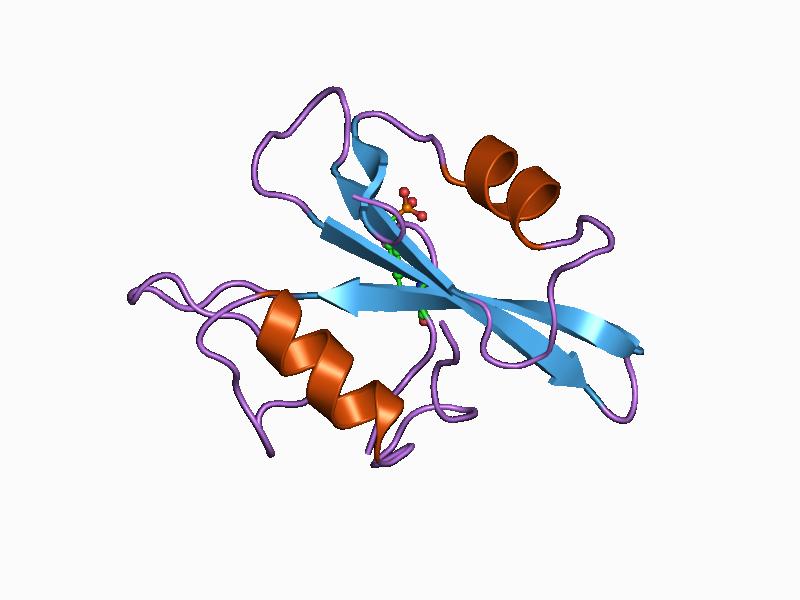
Dectin 1
C-type lectin domain family 7 member A or Dectin-1 is a protein that in humans is encoded by the ''CLEC7A'' gene. CLEC7A is a member of the C-type lectin/C-type lectin-like domain (CTL/CTLD) superfamily. The encoded glycoprotein is a small type II membrane receptor with an extracellular C-type lectin-like domain fold and a cytoplasmic domain with a partial immunoreceptor tyrosine-based activation motif. It functions as a pattern-recognition receptor for a variety of β-1,3-linked and β-1,6-linked glucans from fungi and plants, and in this way plays a role in innate immune response. Expression is found on myeloid dendritic cells, monocytes, macrophages and B cells. Alternate transcriptional splice variants, encoding different isoforms, have been characterized. This gene is closely linked to other CTL/CTLD superfamily members on chromosome 12p13 in the natural killer gene complex region. Structure Dectin-1 is a transmembrane protein containing an immunoreceptor tyrosine-based ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine Phosphorylation
Tyrosine phosphorylation is the addition of a phosphate (PO43−) group to the amino acid tyrosine on a protein. It is one of the main types of protein phosphorylation. This transfer is made possible through enzymes called tyrosine kinases. Tyrosine phosphorylation is a key step in signal transduction and the regulation of enzymatic activity. History In the summer of 1979, studies of polyomavirus middle T and v-Src associated kinase activities led to the discovery of tyrosine phosphorylation as a new type of protein modification. Following the 1979 discovery that Src is a tyrosine kinase, the number of known distinct tyrosine kinases grew rapidly, accelerated by the advent of rapid DNA sequencing technology and PCR. About one year later, researchers discovered an important role for tyrosine phosphorylation in growth factor signaling and proliferation, and by extension in oncogenesis through hijacking of growth factor tyrosine phosphorylation signaling pathways. In 1990 recepto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interleukin 2
Interleukin-2 (IL-2) is an interleukin, a type of cytokine signaling molecule in the immune system. It is a 15.5–16 kDa protein that regulates the activities of white blood cells (leukocytes, often lymphocytes) that are responsible for immunity. IL-2 is part of the body's natural response to microbial infection, and in discriminating between foreign ("non-self") and "self". IL-2 mediates its effects by binding to IL-2 receptors, which are expressed by lymphocytes. The major sources of IL-2 are activated CD4+ T cells and activated CD8+ T cells. IL-2 receptor IL-2 is a member of a cytokine family, each member of which has a four alpha helix bundle; the family also includes IL-4, IL-7, IL-9, IL-15 and IL-21. IL-2 signals through the IL-2 receptor, a complex consisting of three chains, termed alpha (CD25), beta (CD122) and gamma ( CD132). The gamma chain is shared by all family members. The IL-2 receptor (IL-2R) α subunit binds IL-2 with low affinity (Kd~ 10� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interleukin 6
Interleukin 6 (IL-6) is an interleukin that acts as both a pro-inflammatory cytokine and an anti-inflammatory myokine. In humans, it is encoded by the ''IL6'' gene. In addition, osteoblasts secrete IL-6 to stimulate osteoclast formation. Smooth muscle cells in the tunica media of many blood vessels also produce IL-6 as a pro-inflammatory cytokine. IL-6's role as an anti-inflammatory myokine is mediated through its inhibitory effects on TNF-alpha and IL-1 and its activation of IL-1ra and IL-10. There is some early evidence that IL-6 can be used as an inflammatory marker for severe COVID-19 infection with poor prognosis, in the context of the wider coronavirus pandemic. Function Immune system IL-6 is secreted by macrophages in response to specific microbial molecules, referred to as pathogen-associated molecular patterns ( PAMPs). These PAMPs bind to an important group of detection molecules of the innate immune system, called pattern recognition receptors (PRRs), incl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interleukin 23
Interleukin 23 (IL-23) is a heterodimeric cytokine composed of an IL-12B (IL-12p40) subunit (which is shared with IL-12) and an IL-23A (IL-23p19) subunit. IL-23 is part of the IL-12 family of cytokines. The functional receptor for IL-23 (the IL-23 receptor) consists of a heterodimer between IL-12Rβ1 and IL-23R. Discovery IL-23 was first described by Robert Kastelein and colleagues at the DNAX research institute using a combination of computational, biochemical and cellular immunology approaches. Function IL-23 is an inflammatory cytokine. It has been shown to be a key cytokine for T helper type 17 cell (Th17 cell) maintenance and expansion. Polarisation to a Th17 phenotype is triggered by IL-6 and TGF-β, which activate the Th17 transcription factor RORγt. IL-23 stabilises RORγt and thus enables Th17 cells to release their effector cytokines, such as IL-17, IL-21, IL-22 and GM-CSF, which mediate protection against extracellular fungi and bacteria and parti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemokine
Chemokines (), or chemotactic cytokines, are a family of small cytokines or signaling proteins secreted by cells that induce directional movement of leukocytes, as well as other cell types, including endothelial and epithelial cells. In addition to playing a major role in the activation of host immune responses, chemokines are important for biological processes, including morphogenesis and wound healing, as well as in the pathogenesis of diseases like cancers. Cytokine proteins are classified as chemokines according to behavior and structural characteristics. In addition to being known for mediating chemotaxis, chemokines are all approximately 8-10 kilodaltons in mass and have four cysteine residues in conserved locations that are key to forming their 3-dimensional shape. These proteins have historically been known under several other names including the ''SIS family of cytokines'', ''SIG family of cytokines'', ''SCY family of cytokines'', ''Platelet factor-4 superfamily'' or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytokine
Cytokines are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in autocrine, paracrine and endocrine signaling as immunomodulating agents. Cytokines include chemokines, interferons, interleukins, lymphokines, and tumour necrosis factors, but generally not hormones or growth factors (despite some overlap in the terminology). Cytokines are produced by a broad range of cells, including immune cells like macrophages, B lymphocytes, T lymphocytes and mast cells, as well as endothelial cells, fibroblasts, and various stromal cells; a given cytokine may be produced by more than one type of cell. They act through cell surface receptors and are especially important in the immune system; cytokines modulate the balance between humoral and cell-based immune responses, and they regulate the maturation, growth, and res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a protein complex that controls transcription of DNA, cytokine production and cell survival. NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, heavy metals, ultraviolet irradiation, oxidized LDL, and bacterial or viral antigens. NF-κB plays a key role in regulating the immune response to infection. Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development. NF-κB has also been implicated in processes of synaptic plasticity and memory. Discovery NF-κB was discovered by Ranjan Sen in the lab of Nobel laureate David Baltimore via its interaction with an 11-base pair sequence in the immunoglobulin light-chain enhancer in B cells. Later work by Alexander Poltorak and Bruno Lemaitre in mice and ''Drosophila'' frui ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TRAF6
TRAF6 is a TRAF human protein. Function The protein encoded by this gene is a member of the TNF receptor associated factor (TRAF) protein family. TRAF proteins are associated with, and mediate signal transduction from members of the TNF receptor superfamily. This protein mediates the signaling not only from the members of the TNF receptor superfamily, but also from the members of the Toll/IL-1 family. Signals from receptors such as CD40, TNFSF11/TRANCE/RANKL and IL-1 have been shown to be mediated by this protein. This protein also interacts with various protein kinases including IRAK1/IRAK, SRC and PKCzeta, which provides a link between distinct signaling pathways. This protein functions as a signal transducer in the NF-kappaB pathway that activates IkappaB kinase (IKK) in response to proinflammatory cytokines. The interaction of this protein with UBE2N/UBC13, and UBE2V1/UEV1A, which are ubiquitin conjugating enzymes catalyzing the formation of polyubiquitin chains, has been ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CARD-CC Family
The CARD-CC protein family is defined by an evolutionary conserved CARD and a coiled-coil (CC) domain. Coiled-coils (CC) act as oligomerization domains for many proteins such as structural and motor proteins, and transcription factors. This means that monomers are converted to macromolecular complexes by polymerization. The protein family is ancient and can be found as far back as Cnidaria, but has almost exclusively been studied in humans and mice. Notably, the protein family is absent in insects and nematodes, which makes it impossible to study its function in the most popular invertebrate model organisms (Drosophila and C. elegans). In humans and other jawed vertebrates, the family consists of CARD9 and the three "CARD-containing MAGUK protein" (CARMA) proteins CARD11 (CARMA1), CARD14 (CARMA2) and CARD10 (CARMA3). Invertebrates only have a CARD9-like ancestral CARD-CC member, and the earliest occurrence of a CARD-CC member with the CARMA domain composition is in the jawless ver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MALT1
Mucosa-associated lymphoid tissue lymphoma translocation protein 1 is a protein that in humans is encoded by the ''MALT1'' gene. It's the human paracaspase. Function Genetic ablation of the paracaspase gene in mice and biochemical studies have shown that paracaspase is a crucial protein for T and B lymphocytes activation. It has an important role in the activation of the transcription factor NF-κB, in the production of interleukin-2 (IL-2) and in T and B lymphocytes proliferation Two alternatively spliced transcript variants encoding different isoforms have been described for this gene. In addition, a role for paracaspase has been shown in the innate immune response mediated by the zymosan receptor Dectin-1 in macrophages and dendritic cells, and in response to the stimulation of certain G protein-coupled receptors. Sequence analysis proposes that paracaspase has an N-terminal death domain, two central immunoglobulin-like domains involved in the binding to the B-cell lymphoma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BCL10
B-cell lymphoma/leukemia 10 is a protein that in humans is encoded by the ''BCL10'' gene. Like BCL2, BCL3, BCL5, BCL6, BCL7A, and BCL9, it has clinical significance in lymphoma. Function Bcl10 was identified by its translocation in a case of mucosa-associated lymphoid tissue (MALT) lymphoma. The protein encoded by this gene contains a caspase recruitment domain (CARD), and has been shown to induce apoptosis and to activate NF-κB. This protein is reported to interact with other CARD and coiled coil domain containing proteins including CARD9, -10, -11 and -14, which are thought to function as upstream regulators in NF-κB signaling. This protein is found to form a complex with the paracaspase MALT1, a protein encoded by another gene known to be translocated in MALT lymphoma. MALT1 and Bcl10 thought to synergize in the activation of NF-κB, and the deregulation of either of them may contribute to the same pathogenetic process that leads to the malignancy. Bcl10 is evolution ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |