|
DNAPL
A dense non-aqueous phase liquid or DNAPL is a denser-than-water NAPL, i.e. a liquid that is both denser than water and is immiscible in or does not dissolve in water. * in situ surfactant flushing * air sparging * heating Most DNAPLs remain denser than water after they are released into the environment (e.g. spilled trichloroethene does not become lighter than water, it will remain denser than water). However, when the DNAPL is a more complex mixture, the density of the mixture can change over time as the mixture interacts with the natural environment. As an example, a mixture of trichloroethene and cutting oil may be released and originally be denser than water—a DNAPL. As the mixture of trichloroethene and oil is leached by groundwater, the trichloroethene may preferentially leach out of the oil and the mixture may become less dense than water and become buoyant (e.g. the liquid may become an LNAPL). Similarly changes can be seen at some coal gasification Coal gasification is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Light Non-aqueous Phase Liquid
A light non-aqueous phase liquid (LNAPL) is a groundwater contaminant that is not soluble in water and has lower density than water, in contrast to a DNAPL which has higher density than water. Once a LNAPL infiltrates the ground, it will stop at the height of the water table because the LNAPL is less dense than water. Efforts to locate and remove LNAPLs is relatively less expensive and easier than for DNAPLs because LNAPLs float on top of the water in the underground water table. Examples of LNAPLs are benzene, toluene, xylene, and other hydrocarbons. See also * DNAPL * LNAPL transmissivity External linksLNAPL Definitionfrom the USGS The United States Geological Survey (USGS), formerly simply known as the Geological Survey, is a scientific agency of the United States government. The scientists of the USGS study the landscape of the United States, its natural resources, a ... Water pollution Water chemistry Hydrogeology {{Watersupply-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LNAPL
A light non-aqueous phase liquid (LNAPL) is a groundwater contaminant that is not soluble in water and has lower density than water, in contrast to a DNAPL which has higher density than water. Once a LNAPL infiltrates the ground, it will stop at the height of the water table because the LNAPL is less dense than water. Efforts to locate and remove LNAPLs is relatively less expensive and easier than for DNAPLs because LNAPLs float on top of the water in the underground water table. Examples of LNAPLs are benzene, toluene, xylene, and other hydrocarbons. See also * DNAPL * LNAPL transmissivity External linksLNAPL Definitionfrom the USGS The United States Geological Survey (USGS), formerly simply known as the Geological Survey, is a scientific agency of the United States government. The scientists of the USGS study the landscape of the United States, its natural resources, a ... Water pollution Water chemistry Hydrogeology {{Watersupply-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
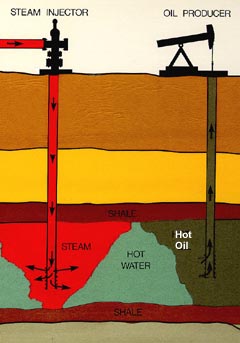
Groundwater Remediation
Groundwater remediation is the process that is used to treat polluted groundwater by removing the pollutants or converting them into harmless products. Groundwater is water present below the ground surface that saturates the pore space in the subsurface. Globally, between 25 per cent and 40 per cent of the world's drinking water is drawn from boreholes and dug wells. Groundwater is also used by farmers to irrigate crops and by industries to produce everyday goods. Most groundwater is clean, but groundwater can become polluted, or contaminated as a result of human activities or as a result of natural conditions. The many and diverse activities of humans produce innumerable waste materials and by-products. Historically, the disposal of such waste have not been subject to many regulatory controls. Consequently, waste materials have often been disposed of or stored on land surfaces where they percolate into the underlying groundwater. As a result, the contaminated groundwater is unsui ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NAPL
Non-aqueous phase liquids, or NAPLs, are organic liquid contaminants that do not dissolve in, or easily mix with, water (hydrophobic), like oil, gasoline and petroleum products. NAPLs tend to contaminate soil and groundwaters for very long period of time: they are persistent organic pollutants (POPs). Many common groundwater contaminants such as chlorinated solvents and many petroleum products enter the subsurface in nonaqueous-phase solutions. They do not mix readily with water and therefore flow separately from ground water. If the NAPL is denser than water, like trichloroethylene, it is called DNAPL (Dense NAPL) and will tend to sink once it reaches the water table. If it is lighter, like gasoline, it is called LNAPL (Light NAPL) and will tend to float on the water table. National Research Council, 1997 See also * DNAPL * LNAPL Notes References * National Research Council, 1997Innovations in ground water and soil cleanup--From concept to commercialization Washington ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extra Heavy Crude Oil
Heavy crude oil (or extra heavy crude oil) is highly-viscous oil that cannot easily flow from production wells under normal reservoir conditions. It is referred to as "heavy" because its density or specific gravity is higher than that of light crude oil. Heavy crude oil has been defined as any liquid petroleum with an API gravity less than 20°. Physical properties that differ between heavy crude oils and lighter grades include higher viscosity and specific gravity, as well as higher molecular weight hydrocarbon composition. In 2010, the World Energy Council defined extra heavy oil as crude oil having a gravity of less than 10° and a reservoir viscosity of more than 10,000 centipoises. When reservoir viscosity measurements are not available, extra-heavy oil is considered by the WEC to have a lower limit of 4° API. In other words, oil with a density greater than 1000 kg/m3 (or a specific gravity greater than 1) and a reservoir viscosity of more than 10,000 centipois ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trichloroethylene
The chemical compound trichloroethylene is a halocarbon commonly used as an industrial solvent. It is a clear, colourless non-flammable liquid with a chloroform-like sweet smell. It should not be confused with the similar 1,1,1-trichloroethane, which is commonly known as ''chlorothene''. The IUPAC name is trichloroethene. Industrial abbreviations include TCE, trichlor, Trike, Tricky and tri. It has been sold under a variety of trade names. Under the trade names Trimar and Trilene, trichloroethylene was used as a volatile anesthetic and as an inhaled obstetrical analgesic in millions of patients. Groundwater and drinking water contamination from industrial discharge including trichloroethylene is a major concern for human health and has precipitated numerous incidents and lawsuits. History Pioneered by Imperial Chemical Industries in Britain, its development was hailed as an anesthetic revolution. Originally thought to possess less hepatotoxicity than chloroform, and without th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
World War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the vast majority of the world's countries—including all of the great powers—forming two opposing military alliances: the Allies and the Axis powers. World War II was a total war that directly involved more than 100 million personnel from more than 30 countries. The major participants in the war threw their entire economic, industrial, and scientific capabilities behind the war effort, blurring the distinction between civilian and military resources. Aircraft played a major role in the conflict, enabling the strategic bombing of population centres and deploying the only two nuclear weapons ever used in war. World War II was by far the deadliest conflict in human history; it resulted in 70 to 85 million fatalities, mostly among civilians. Tens of millions died due to genocides (including the Holocaust), starvation, ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coal Gasification
Coal gasification is the process of producing syngas—a mixture consisting primarily of carbon monoxide (CO), hydrogen (H2), carbon dioxide (CO2), methane (CH4), and water vapour (H2O)—from coal and water, air and/or oxygen. Historically, coal was gasified to produce coal gas, also known as "town gas". Coal gas is combustible and was used for heating and municipal lighting, before the advent of large-scale extraction of natural gas from oil wells. In current practice, large-scale coal gasification installations are primarily for electricity generation (both in conventional thermal power stations and molten carbonate fuel cell power stations), or for production of chemical feedstocks. The hydrogen obtained from coal gasification can be used for various purposes such as making ammonia, powering a hydrogen economy, or upgrading fossil fuels. Alternatively, coal-derived syngas can be converted into transportation fuels such as gasoline and diesel through additional treatment, or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reductive Dechlorination
In Organochlorine compound, organochlorine chemistry, reductive dechlorination describes any chemical reaction which Bond cleavage, cleaves the covalent bond between carbon and chlorine via reductants, to release chloride ions. Many modalities have been implemented, depending on the application. Reductive dechlorination is often applied to remediation of chlorinated Pesticide, pesticides or dry cleaning solvents. It is also used occasionally in the organic synthesis, synthesis of organic compounds, e.g. as pharmaceuticals. Chemical Dechlorination is a common reaction in organic Organic synthesis, synthesis. Usually only stoichiometric amounts of dechlorinating agent are used. A classic Ullmann reaction is the conversion of 2-Chloronitrobenzene, 2-chloronitrobenzene into 2,2'-dinitrobiphenyl with a copper - bronze alloy. Other examples: *Zerovalent iron *Organophosphorus compound, Organophosphorus(III) compounds effect gentle dechlorinations. The products are alkenes and phosphoru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Persulfate
A persulfate (sometimes known as peroxysulfate or peroxodisulfate) is a compound containing the anions or . The anion contains one peroxide group per sulfur center, whereas in , the peroxide group bridges the sulfur atoms. In both cases, sulfur adopts the normal tetrahedral geometry typical for the S(VI) oxidation state. These salts are strong oxidizers. Ions * Peroxomonosulfate ion, * Peroxydisulfate Acids * Peroxymonosulfuric acid (Caro's acid), H2SO5 * Peroxydisulfuric acid, H2S2O8 Example salts * Sodium peroxomonosulfate, Na2SO5 * Potassium peroxymonosulfate, KHSO5 * Sodium persulfate (sodium peroxydisulfate), Na2S2O8 * Ammonium persulfate (ammonium peroxydisulfate), (NH4)2S2O8 * Potassium persulfate Potassium persulfate is the inorganic compound with the formula K2 S2O8. Also known as potassium peroxydisulfate, it is a white solid that is sparingly soluble in cold water, but dissolves better in warm water. This salt is a powerful oxidant, c ... (potassium peroxyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lower atmosphere to (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the latter, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation. Ozone's odour is reminiscent of chlorine, and detectable by many people at concentrations of as little as in air. Ozone's O3 structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetic. In standard conditions, ozone is a pale blue gas that condenses at cryogenic temperatures to a dark blue liquid and finally a violet-black soli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or " high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a stabilizer in a weakly acidic solution in a dark bottle to block light. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases. Properties The boiling poi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |