|
Diffusion Limited Enzyme
A diffusion-limited enzyme catalyses a reaction so efficiently that the rate limiting step is that of substrate diffusion into the active site, or product diffusion out. This is also known as kinetic perfection or catalytic perfection. Since the rate of catalysis of such enzymes is set by the diffusion-controlled reaction, it therefore represents an intrinsic, physical constraint on evolution (a maximum peak height in the fitness landscape). Diffusion limited perfect enzymes are very rare. Most enzymes catalyse their reactions to a rate that is 1,000-10,000 times slower than this limit. This is due to both the chemical limitations of difficult reactions, and the evolutionary limitations that such high reaction rates do not confer any extra fitness. History The theory of diffusion-controlled reaction was originally utilized by R.A. Alberty, Gordon Hammes, and Manfred Eigen to estimate the upper limit of enzyme-substrate reaction. According to their estimation, the upper limi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activity Distribution Graph
Activity may refer to: * Action (philosophy), in general * Human activity: human behavior, in sociology behavior may refer to all basic human actions, economics may study human economic activities and along with cybernetics and psychology may study their modulation * Recreation, or activities of leisure * The Aristotelian concept of energeia, Latinized as ''actus'' * Activity (UML), a major task in Unified Modeling Language * ''Activity'', the rate of catalytic activity, such as enzyme activity (enzyme assay), in physical chemistry and enzymology * Thermodynamic activity, the effective concentration of a solute for the purposes of mass action * Activity (project management) * Activity, the number of radioactive decays per second * Activity (software engineering) * Activity (soil mechanics) * , an aircraft carrier of the Royal Navy * "Activity", a song by Way Out West from ''Intensify'' * Cultural activities, activities referred to culture. See also * Activity theory, a learni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substrate (biochemistry)
In chemistry, the term substrate is highly context-dependent. Broadly speaking, it can refer either to a chemical species being observed in a chemical reaction, or to a surface on which other chemical reactions or microscopy are performed. In the former sense, a reagent is added to the ''substrate'' to generate a product through a chemical reaction. The term is used in a similar sense in synthetic and organic chemistry, where the substrate is the chemical of interest that is being modified. In biochemistry, an enzyme substrate is the material upon which an enzyme acts. When referring to Le Chatelier's principle, the substrate is the reagent whose concentration is changed. ;Spontaneous reaction : :*Where S is substrate and P is product. ;Catalysed reaction : :*Where S is substrate, P is product and C is catalyst. In the latter sense, it may refer to a surface on which other chemical reactions are performed or play a supporting role in a variety of spectroscopic and microsc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffusion-controlled Reaction
Diffusion-controlled (or diffusion-limited) reactions are reactions in which the reaction rate is equal to the rate of transport of the reactants through the reaction medium (usually a solution). The process of chemical reaction can be considered as involving the diffusion of reactants until they encounter each other in the right stoichiometry and form an activated complex which can form the product species. The observed rate of chemical reactions is, generally speaking, the rate of the slowest or "rate determining" step. In diffusion controlled reactions the formation of products from the activated complex is much faster than the diffusion of reactants and thus the rate is governed by collision frequency. Diffusion control is rare in the gas phase, where rates of diffusion of molecules are generally very high. Diffusion control is more likely in solution where diffusion of reactants is slower due to the greater number of collisions with solvent molecules. Reactions where the acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triosephosphate Isomerase
Triose-phosphate isomerase (TPI or TIM) is an enzyme () that catalyzes the reversible interconversion of the triose phosphate isomers dihydroxyacetone phosphate and D-glyceraldehyde 3-phosphate. TPI plays an important role in glycolysis and is essential for efficient energy production. TPI has been found in nearly every organism searched for the enzyme, including animals such as mammals and insects as well as in fungi, plants, and bacteria. However, some bacteria that do not perform glycolysis, like ureaplasmas, lack TPI. In humans, deficiencies in TPI are associated with a progressive, severe neurological disorder called triose phosphate isomerase deficiency. Triose phosphate isomerase deficiency is characterized by chronic hemolytic anemia. While there are various mutations that cause this disease, most include the replacement of glutamic acid at position 104 with an aspartic acid. Triose phosphate isomerase is a highly efficient enzyme, performing the reaction billions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superoxide Dismutase
Superoxide dismutase (SOD, ) is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide () radical into ordinary molecular oxygen (O2) and hydrogen peroxide (). Superoxide is produced as a by-product of oxygen metabolism and, if not regulated, causes many types of cell damage. Hydrogen peroxide is also damaging and is degraded by other enzymes such as catalase. Thus, SOD is an important antioxidant defense in nearly all living cells exposed to oxygen. One exception is '' Lactobacillus plantarum'' and related lactobacilli, which use a different mechanism to prevent damage from reactive . Chemical reaction SODs catalyze the disproportionation of superoxide: : 2 HO2 → O2 + H2O2 In this way, is converted into two less damaging species. The pathway by which SOD-catalyzed dismutation of superoxide may be written, for Cu,Zn SOD, with the following reactions: * Cu2+-SOD + → Cu+-SOD + O2 (reduction of copper; oxidation of superoxide) * Cu+-S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fumarase
Fumarase (or fumarate hydratase) is an enzyme () that catalyzes the reversible hydration/dehydration of fumarate to malate. Fumarase comes in two forms: mitochondrial and cytosolic. The mitochondrial isoenzyme is involved in the Krebs cycle and the cytosolic isoenzyme is involved in the metabolism of amino acids and fumarate. Subcellular localization is established by the presence of a signal sequence on the amino terminus in the mitochondrial form, while subcellular localization in the cytosolic form is established by the absence of the signal sequence found in the mitochondrial variety. This enzyme participates in 2 metabolic pathways: citric acid cycle and reductive citric acid cycle (CO2 fixation), and is also important in renal cell carcinoma. Mutations in this gene have been associated with the development of leiomyomas in the skin and uterus in combination with renal cell carcinoma ( HLRCC syndrome). Nomenclature This enzyme belongs to the family of lyases, specifi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome C Peroxidase
Cytochrome ''c'' peroxidase, or CCP, is a water-soluble heme-containing enzyme of the peroxidase family that takes reducing equivalents from cytochrome ''c'' and reduces hydrogen peroxide to water: :CCP + H2O2 + 2 ferrocytochrome ''c'' + 2H+ → CCP + 2H2O + 2 ferricytochrome ''c'' CCP can be derived from aerobically grown yeast strains and can be isolated in both native and recombinant forms with high yield from ''Saccharomyces cerevisiae.'' The enzyme’s primary function is to eliminate toxic radical molecules produced by the cell which are harmful to biological systems. It works to maintain low concentration levels of hydrogen peroxide, which is generated by the organism naturally through incomplete oxygen reduction. When glucose levels in fast growing yeast strains are exhausted, the cells turn to respiration which raises the concentration of mitochondrial H2O2. In addition to its peroxidase activity, it acts as a sensor and a signaling molecule to exogenous H2O2, which acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide Dehydrogenase
In enzymology, carbon monoxide dehydrogenase (CODH) () is an enzyme that catalyzes the chemical reaction :CO + H2O + A \rightleftharpoons CO2 + AH2 The chemical process catalyzed by carbon monoxide dehydrogenase is similar to the water-gas shift reaction. The 3 substrates of this enzyme are CO, H2O, and A, whereas its two products are CO2 and AH2. A variety of electron donors/receivers (Shown as "A" and "AH2" in the reaction equation above) are observed in micro-organisms which utilize CODH. Several examples of electron transfer cofactors has been proposed, including Ferredoxin, NADP+/NADPH and flavoprotein complexes like flavin adenine dinucleotide (FAD) as well as hydrogenases. CODHs support the metabolisms of diverse prokaryotes, including methanogens, aerobic carboxidotrophs, acetogens, sulfate-reducers, and hydrogenogenic bacteria. The bidirectional reaction catalyzed by CODH plays a role in the carbon cycle allowing organisms to both make use of CO as a source o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonic Anhydrase
The carbonic anhydrases (or carbonate dehydratases) () form a family of enzymes that catalyze the interconversion between carbon dioxide and water and the dissociated ions of carbonic acid (i.e. bicarbonate and hydrogen ions). The active site of most carbonic anhydrases contains a zinc ion. They are therefore classified as metalloenzymes. The enzyme maintains acid-base balance and helps transport carbon dioxide. Carbonic anhydrase helps maintain acid–base homeostasis, regulate pH, and fluid balance. Depending on its location, the role of the enzyme changes slightly. For example, carbonic anhydrase produces acid in the stomach lining. In the kidney, the control of bicarbonate ions influences the water content of the cell. The control of bicarbonate ions also influences the water content in the eyes. Inhibitors of carbonic anhydrase are used to treat glaucoma, the excessive build-up of water in the eyes. Blocking this enzyme shifts the fluid balance in the eyes to reduce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalase
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen (such as bacteria, plants, and animals) which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting the cell from oxidative damage by reactive oxygen species (ROS). Catalase has one of the highest turnover numbers of all enzymes; one catalase molecule can convert millions of hydrogen peroxide molecules to water and oxygen each second. Catalase is a tetramer of four polypeptide chains, each over 500 amino acids long. It contains four iron-containing heme groups that allow the enzyme to react with hydrogen peroxide. The optimum pH for human catalase is approximately 7, and has a fairly broad maximum: the rate of reaction does not change appreciably between pH 6.8 and 7.5. The pH optimum for other catalases varies between 4 and 11 depending on the species. The optimum temperature also varies by species. Structure Human catalase forms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-lactamase
Beta-lactamases, (β-lactamases) are enzymes () produced by bacteria that provide multi-resistance to beta-lactam antibiotics such as penicillins, cephalosporins, cephamycins, monobactams and carbapenems ( ertapenem), although carbapenems are relatively resistant to beta-lactamase. Beta-lactamase provides antibiotic resistance by breaking the antibiotics' structure. These antibiotics all have a common element in their molecular structure: a four-atom ring known as a beta-lactam (β-lactam) ring. Through hydrolysis, the enzyme lactamase breaks the β-lactam ring open, deactivating the molecule's antibacterial properties. Beta-lactam antibiotics are typically used to target a broad spectrum of gram-positive and gram-negative bacteria. Beta-lactamases produced by gram-negative bacteria are usually secreted, especially when antibiotics are present in the environment. Structure The structure of a ''Streptomyces'' serine β-lactamase (SBLs) is given by . The alpha-beta f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
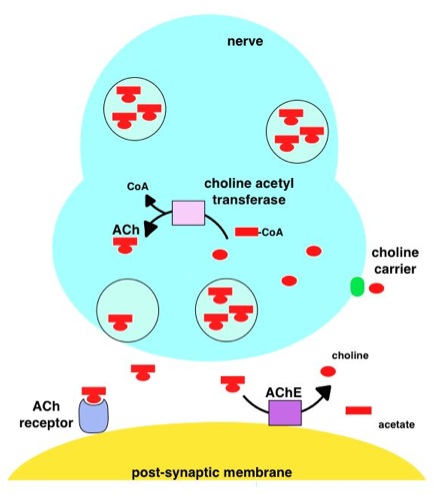
Acetylcholinesterase
Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters: : acetylcholine + H2O = choline + acetate It is found at mainly neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate synaptic transmission. It belongs to the carboxylesterase family of enzymes. It is the primary target of inhibition by organophosphorus compounds such as nerve agents and pesticides. Enzyme structure and mechanism AChE is a hydrolase that hydrolyzes choline esters. It has a very high catalytic activity—each molecule of AChE degrades about 25,000 molecules of acetylcholine (ACh) per second, approaching the limit allowed by diffusion of the substrate. The active site of AChE comprises 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |