|
DEP Domain
In molecular biology, the DEP domain (Dishevelled, Egl-10 and Pleckstrin domain) is a globular protein domain of about 80 amino acids that is found in over 50 proteins involved in G-protein signalling pathways. It was named after the three proteins it was initially found in: *Dishevelled (Dsh and Dvl), which plays a key role in the transduction of the Wg/Wnt signal from the cell surface to the nucleus; it is a segment polarity protein required to establish coherent arrays of polarised cells and segments in embryos, and plays a role in wingless signalling. *Egl-10, which regulates G-protein signalling in the central nervous system in RGS9. *Pleckstrin, the major substrate of protein kinase C in platelets; Pleckstrin contains two PH domains flanking the DEP domain. Mammalian regulators of G-protein signalling also contain these domains, and regulate signal transduction by increasing the GTPase activity of G-protein alpha subunits, thereby driving them into their inactive GDP-bou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
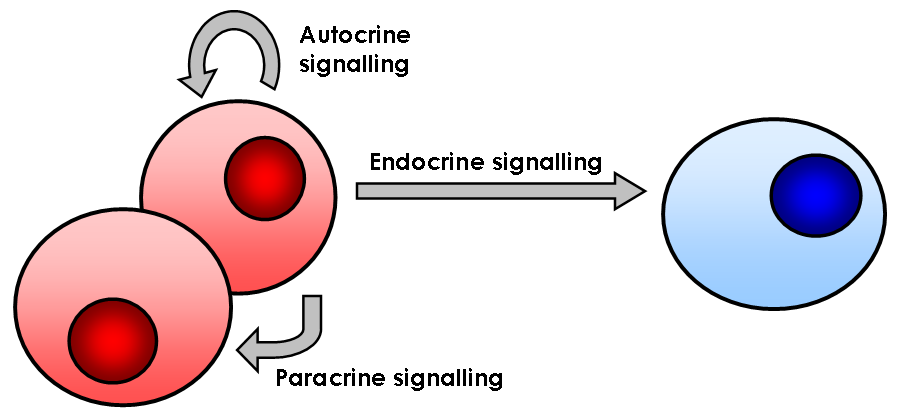
Cell Signaling
In biology, cell signaling (cell signalling in British English) or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. Cell signaling is a fundamental property of all cellular life in prokaryotes and eukaryotes. Signals that originate from outside a cell (or extracellular signals) can be physical agents like mechanical pressure, voltage, temperature, light, or chemical signals (e.g., small molecules, peptides, or gas). Cell signaling can occur over short or long distances, and as a result can be classified as autocrine, juxtacrine, intracrine, paracrine, or endocrine. Signaling molecules can be synthesized from various biosynthetic pathways and released through passive or active transports, or even from cell damage. Receptors play a key role in cell signaling as they are able to detect chemical signals or physical stimuli. Receptors are generally proteins located on the cell surface or within th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein-coupled
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times. Text was copied from this source, which is available under Attribution 2.5 Generic (CC BY 2.5) license. Ligands can bind either to extracellular N-terminus and loops (e.g. glutamate receptors) or to the binding site within transmembrane helices (Rhodopsin-like family). They are all activated by agonists although a spontaneous auto-activation of an empty receptor can also be observed. G protein-coupled receptors are found only in eukaryotes, including yeast, choanoflagellates, and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GTPase
GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved P-loop "G domain", a protein domain common to many GTPases. Functions GTPases function as molecular switches or timers in many fundamental cellular processes. Examples of these roles include: * Signal transduction in response to activation of cell surface receptors, including transmembrane receptors such as those mediating taste, smell and vision. * Protein biosynthesis (a.k.a. translation) at the ribosome. * Regulation of cell differentiation, proliferation, division and movement. * Translocation of proteins through membranes. * Transport of vesicles within the cell, and vesicle-mediated secretion and uptake, through GTPase control of vesicle coat assembly. GTPases are active when bound to GTP and inactive when bound to GDP. In the generalized r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Signal Transduction
Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events, most commonly protein phosphorylation catalyzed by protein kinases, which ultimately results in a cellular response. Proteins responsible for detecting stimuli are generally termed receptors, although in some cases the term sensor is used. The changes elicited by ligand binding (or signal sensing) in a receptor give rise to a biochemical cascade, which is a chain of biochemical events known as a signaling pathway. When signaling pathways interact with one another they form networks, which allow cellular responses to be coordinated, often by combinatorial signaling events. At the molecular level, such responses include changes in the transcription or translation of genes, and post-translational and conformational changes in proteins, as well as changes in their location. These molecular events are the basic mechanisms controlling cell gro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mammalia
Mammals () are a group of vertebrate animals constituting the class Mammalia (), characterized by the presence of mammary glands which in females produce milk for feeding (nursing) their young, a neocortex (a region of the brain), fur or hair, and three middle ear bones. These characteristics distinguish them from reptiles (including birds) from which they diverged in the Carboniferous, over 300 million years ago. Around 6,400 extant species of mammals have been described divided into 29 orders. The largest orders, in terms of number of species, are the rodents, bats, and Eulipotyphla ( hedgehogs, moles, shrews, and others). The next three are the Primates (including humans, apes, monkeys, and others), the Artiodactyla (cetaceans and even-toed ungulates), and the Carnivora (cats, dogs, seals, and others). In terms of cladistics, which reflects evolutionary history, mammals are the only living members of the Synapsida (synapsids); this clade, toget ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pleckstrin Homology Domain
Pleckstrin homology domain (PH domain) or (PHIP) is a protein domain of approximately 120 amino acids that occurs in a wide range of proteins involved in intracellular signaling or as constituents of the cytoskeleton. This domain can bind phosphatidylinositol lipids within biological membranes (such as phosphatidylinositol (3,4,5)-trisphosphate and phosphatidylinositol (4,5)-bisphosphate), and proteins such as the βγ-subunits of heterotrimeric G proteins, and protein kinase C. Through these interactions, PH domains play a role in recruiting proteins to different membranes, thus targeting them to appropriate cellular compartments or enabling them to interact with other components of the signal transduction pathways. Lipid binding specificity Individual PH domains possess specificities for phosphoinositides phosphorylated at different sites within the inositol ring, e.g., some bind phosphatidylinositol (4,5)-bisphosphate but not phosphatidylinositol (3,4,5)-trisphosphate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platelets
Platelets, also called thrombocytes (from Greek θρόμβος, "clot" and κύτος, "cell"), are a component of blood whose function (along with the coagulation factors) is to react to bleeding from blood vessel injury by clumping, thereby initiating a blood clot. Platelets have no cell nucleus; they are fragments of cytoplasm that are derived from the megakaryocytes of the bone marrow or lung, which then enter the circulation. Platelets are found only in mammals, whereas in other vertebrates (e.g. birds, amphibians), thrombocytes circulate as intact mononuclear cells. One major function of platelets is to contribute to hemostasis: the process of stopping bleeding at the site of interrupted endothelium. They gather at the site and, unless the interruption is physically too large, they plug the hole. First, platelets attach to substances outside the interrupted endothelium: ''adhesion''. Second, they change shape, turn on receptors and secrete chemical messengers: ''ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Kinase C
In cell biology, Protein kinase C, commonly abbreviated to PKC (EC 2.7.11.13), is a family of protein kinase enzymes that are involved in controlling the function of other proteins through the phosphorylation of hydroxyl groups of serine and threonine amino acid residues on these proteins, or a member of this family. PKC enzymes in turn are activated by signals such as increases in the concentration of diacylglycerol (DAG) or calcium ions (Ca2+). Hence PKC enzymes play important roles in several signal transduction cascades. In biochemistry, the PKC family consists of fifteen isozymes in humans. They are divided into three subfamilies, based on their second messenger requirements: conventional (or classical), novel, and atypical. Conventional (c)PKCs contain the isoforms α, βI, βII, and γ. These require Ca2+, DAG, and a phospholipid such as phosphatidylserine for activation. Novel (n)PKCs include the δ, ε, η, and θ isoforms, and require DAG, but do not require Ca2+ f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Substrate
In chemistry, the term substrate is highly context-dependent. Broadly speaking, it can refer either to a chemical species being observed in a chemical reaction, or to a surface on which other chemical reactions or microscopy are performed. In the former sense, a reagent is added to the ''substrate'' to generate a product through a chemical reaction. The term is used in a similar sense in synthetic and organic chemistry, where the substrate is the chemical of interest that is being modified. In biochemistry, an enzyme substrate is the material upon which an enzyme acts. When referring to Le Chatelier's principle, the substrate is the reagent whose concentration is changed. ;Spontaneous reaction : :*Where S is substrate and P is product. ;Catalysed reaction : :*Where S is substrate, P is product and C is catalyst. In the latter sense, it may refer to a surface on which other chemical reactions are performed or play a supporting role in a variety of spectroscopic and microsco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pleckstrin
Pleckstrin is a protein found in platelets. The name derives from platelet and leukocyte C kinase substrate and the KSTR string of amino acids. It is the source of the name pleckstrin homology domain Pleckstrin homology domain (PH domain) or (PHIP) is a protein domain of approximately 120 amino acids that occurs in a wide range of proteins involved in intracellular signaling or as constituents of the cytoskeleton. This domain can bind phosp .... External links * Proteins {{biochemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RGS9
Regulator of G-protein signalling 9, also known as RGS9, is a human gene, which codes for a protein involved in regulation of signal transduction inside cells. Members of the RGS family, such as RGS9, are signaling proteins that suppress the activity of G proteins by promoting their deactivation. upplied by OMIMref name="entrez" /> There are two splice isoforms of RGS9 with quite different properties and patterns of expression. RGS9-1 is mainly found in the eye and is involved in regulation of phototransduction in rod and cone cells of the retina, while RGS9-2 is found in the brain, and regulates dopamine and opioid signaling in the basal ganglia The basal ganglia (BG), or basal nuclei, are a group of subcortical nuclei, of varied origin, in the brains of vertebrates. In humans, and some primates, there are some differences, mainly in the division of the globus pallidus into an extern .... RGS9-2 is of particular interest as the most important RGS protein involved in term ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |