|
Copper(III) Oxide
Copper(III) oxide is a hypothetical inorganic compound with the formula Cu2O3. It has not been isolated as a pure solid. Copper(III) oxides are constituents of cuprate superconductors. Copper(III) is typically stabilized in an ionic environment, e.g. potassium hexafluorocuprate(III) Potassium hexafluorocuprate(III) is an inorganic compound with the chemical formula K3CuF6. It is a green paramagnetic solid, a relatively rare example of a copper(III) compound. Synthesis and structure The compound is prepared by oxidizing th .... References * Chemical encyclopedia / Editorial Board .: Knuniants IL etc. .. - M.: Soviet Encyclopedia, 1990 - V. 2 - 671 s. - . * R. Ripa, Chetyanu I. Inorganic Chemistry. Chemistry of Metals. - M.: Mir, 1972 - V. 2 - 871 s. Copper compounds Hypothetical chemical compounds Transition metal oxides Sesquioxides {{theoretical-chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel(III) Oxide
Nickel (III) oxide is the inorganic compound with the formula Ni2O3. It is not well characterized, and is sometimes referred to as black nickel oxide. Traces of Ni2O3 on nickel surfaces have been mentioned. Nickel (III) oxide has been studied theoretically since the early thirties, supporting its unstable nature at standard temperatures. A nanostructured pure phase of the material was synthesized and stabilized for the first time in 2015 from the reaction of nickel(II) nitrate with sodium hypochlorite and characterized using powder X-ray diffraction and electron microscopy. References Inorganic compounds Catalysts Electrochemistry Transition metal oxides Nickel compounds Non-stoichiometric compounds Sesquioxides {{electrochem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(I) Oxide
Copper(I) oxide or cuprous oxide is the inorganic compound with the formula Cu2O. It is one of the principal oxides of copper, the other being or copper(II) oxide or cupric oxide (CuO). This red-coloured solid is a component of some antifouling paints. The compound can appear either yellow or red, depending on the size of the particles. Copper(I) oxide is found as the reddish mineral cuprite. Preparation Copper(I) oxide may be produced by several methods. Most straightforwardly, it arises via the oxidation of copper metal: : 4 Cu + O2 → 2 Cu2O Additives such as water and acids affect the rate of this process as well as the further oxidation to copper(II) oxides. It is also produced commercially by reduction of copper(II) solutions with sulfur dioxide. Reactions Aqueous cuprous chloride solutions react with base to give the same material. In all cases, the color is highly sensitive to the procedural details. Formation of copper(I) oxide is the basis of the F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
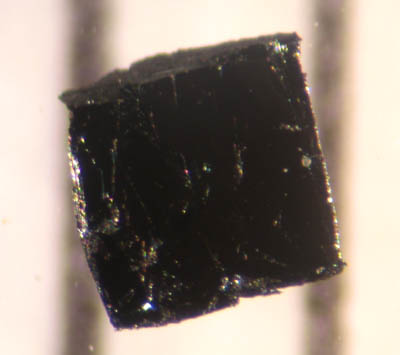
Copper(II) Oxide
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of copper mining and the precursor to many other copper-containing products and chemical compounds. Production It is produced on a large scale by pyrometallurgy, as one stage in extracting copper from its ores. The ores are treated with an aqueous mixture of ammonium carbonate, ammonia, and oxygen to give copper(I) and copper(II) ammine complexes, which are extracted from the solids. These complexes are decomposed with steam to give CuO. It can be formed by heating copper in air at around 300–800°C: : 2 Cu + O2 → 2 CuO For laboratory uses, pure copper(II) oxide is better prepared by heating copper(II) nitrate, copper(II) hydroxide, or basic copper(II) carbonate: : 2 Cu(NO3)2(s) → 2 CuO(s) + 4 NO2(g) + O2(g) (180° ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High-temperature Superconductivity
High-temperature superconductors (abbreviated high-c or HTS) are defined as materials that behave as superconductors at temperatures above , the boiling point of liquid nitrogen. The adjective "high temperature" is only in respect to previously known superconductors, which function at even colder temperatures close to absolute zero. In absolute terms, these "high temperatures" are still far below ambient, and therefore require cooling. The first high-temperature superconductor was discovered in 1986, by IBM researchers Bednorz and Müller, who were awarded the Nobel Prize in Physics in 1987 "for their important break-through in the discovery of superconductivity in ceramic materials". Most high-c materials are type-II superconductors. The major advantage of high-temperature superconductors is that they can be cooled by using liquid nitrogen, as opposed to the previously known superconductors which require expensive and hard-to-handle coolants, primarily liquid helium. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Hexafluorocuprate(III)
Potassium hexafluorocuprate(III) is an inorganic compound with the chemical formula K3CuF6. It is a green paramagnetic solid, a relatively rare example of a copper(III) compound. Synthesis and structure The compound is prepared by oxidizing the mixture of potassium chloride and cuprous chloride with fluorine: :3 KCl + CuCl + 3 F2 → K3CuF6 + 2 Cl2 A variety of analogues are known.R. Hoppe, G. Wingefeld "Zur Kenntnis der Hexafluorocuprate(III)" Zeitschrift für anorganische und allgemeine Chemie 1984, Vol. 519, pages 195–203. The compound reacts with water easily, producing oxygen and copper(II) products. See also *Cuprate(III) Cuprate loosely refers to a material that can be viewed as containing anionic copper complexes. Examples include tetrachloridocuprate ( uCl4sup>2−), the superconductor YBa2Cu3O7, and the organocuprates (e.g., dimethylcuprate u(CH3)2sup>� ... * caesium hexafluorocuprate(IV) References {{Potassium compounds Copper compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper Compounds
Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called ''cuprous'' and ''cupric'', respectively. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological processes. Binary compounds As with other elements, the simplest compounds of copper are binary compounds, i.e. those containing only two elements, the principal examples being oxides, sulfides, and halides. Both cuprous and cupric oxides are known. Among the numerous copper sulfides, important examples include copper(I) sulfide and copper(II) sulfide. Cuprous halides with fluorine, chlorine, bromine, and iodine are known, as are cupric halides with fluorine, chlorine, and bromine. Attempts to prepare copper(II) iodide yield only copper(I) iodide and iodine. :2 Cu2+ + 4 I− → 2 CuI + I2 Coordination chemistry Copper forms coordination complexes with ligands. In aqueous solution, copper(II) exists as . This compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypothetical Chemical Compounds
A hypothesis (plural hypotheses) is a proposed explanation for a phenomenon. For a hypothesis to be a scientific hypothesis, the scientific method requires that one can test it. Scientists generally base scientific hypotheses on previous observations that cannot satisfactorily be explained with the available scientific theories. Even though the words "hypothesis" and "theory" are often used interchangeably, a scientific hypothesis is not the same as a scientific theory. A working hypothesis is a provisionally accepted hypothesis proposed for further research in a process beginning with an educated guess or thought. A different meaning of the term ''hypothesis'' is used in formal logic, to denote the antecedent of a proposition; thus in the proposition "If ''P'', then ''Q''", ''P'' denotes the hypothesis (or antecedent); ''Q'' can be called a consequent. ''P'' is the assumption in a (possibly counterfactual) ''What If'' question. The adjective ''hypothetical'', meaning "havin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal Oxides
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry (the measurable relationship between reactants and chemical equations of a equation or reaction) Oxides are extraordinarily diverse in terms of stoichiometries and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |