|
Copolymer
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called ''bipolymers''. Those obtained from three and four monomers are called ''terpolymers'' and ''quaterpolymers'', respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material. Commercial copolymers include acrylonitrile butadiene styrene (ABS), styrene/butadiene co-polymer (SBR), nitrile rubber, styrene-acrylonitrile, styrene-isoprene-styrene (SIS) and ethylene-vinyl acetate, all of which are formed by chain-growth polymerization. Another production mechanism is step-growth polymerization, which is used to produce the nylon-12/6/66 copolymer of nylon 12, nylon 6 and nylon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copolymers
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called ''bipolymers''. Those obtained from three and four monomers are called ''terpolymers'' and ''quaterpolymers'', respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material. Commercial copolymers include acrylonitrile butadiene styrene (ABS), styrene/butadiene co-polymer (SBR), nitrile rubber, styrene-acrylonitrile, styrene-isoprene-styrene (SIS) and ethylene-vinyl acetate, all of which are formed by chain-growth polymerization. Another production mechanism is step-growth polymerization, which is used to produce the nylon-12/6/66 copolymer of nylon 12, nylon 6 and nylo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
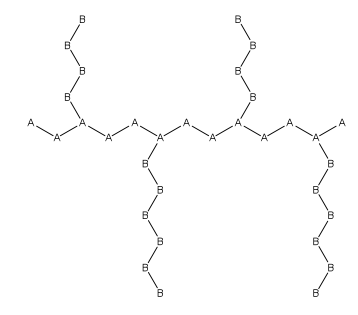
Graft Polymer
In polymer chemistry, graft polymers are segmented copolymers with a linear backbone of one composite and randomly distributed branches of another composite. The picture labeled "graft polymer" shows how grafted chains of species B are covalently bonded to polymer species A. Although the side chains are structurally distinct from the main chain, the individual grafted chains may be homopolymers or copolymers. Graft polymers have been synthesized for many decades and are especially used as impact resistant materials, thermoplastic elastomers, compatibilizers, or emulsifiers for the preparation of stable blends or alloys. One of the better-known examples of a graft polymer is a component used in high impact polystyrene, consisting of a polystyrene backbone with polybutadiene grafted chains. General properties Graft copolymer In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mayo–Lewis Equation
The Mayo–Lewis equation or copolymer equation in polymer chemistry describes the distribution of monomers in a copolymer. It was proposed by Frank R. Mayo and Frederick M. Lewis.''Copolymerization. I. A Basis for Comparing the Behavior of Monomers in Copolymerization; The Copolymerization of Styrene and Methyl Methacrylate'' Frank R. Mayo and Frederick M. Lewis J. Am. Chem. Soc.; 1944; 66(9) pp 1594 - 1601; The equation considers a monomer mix of two components M_1\, and M_2\, and the four different reactions that can take place at the reactive chain end terminating in either monomer (M_1^*\, and M_2^*\,) with their reaction rate constants k\,: :M_1^* + M_1 \xrightarrow M_1M_1^* \, :M_1^* + M_2 \xrightarrow M_1M_2^* \, :M_2^* + M_2 \xrightarrow M_2M_2^* \, :M_2^* + M_1 \xrightarrow M_2M_1^* \, The reactivity ratio for each propagating chain end is defined as the ratio of the rate constant for addition of a monomer of the species already at the chain end to the rate constant for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene-vinyl Acetate
Ethylene-vinyl acetate (EVA), also known as poly (ethylene-vinyl acetate) (PEVA), is the copolymer of ethylene and vinyl acetate. The weight percent of vinyl acetate usually varies from 10 to 40%, with the remainder being ethylene. There are three different types of EVA copolymer, which differ in the vinyl acetate (VA) content and the way the materials are used. The EVA copolymer which is based on a low proportion of VA (approximately up to 4%) may be referred to as vinyl acetate modified polyethylene. It is a copolymer and is processed as a thermoplastic material – just like low density polyethylene. It has some of the properties of a low density polyethylene but increased gloss (useful for film), softness and flexibility. The material is generally considered non-toxic. The EVA copolymer, which is based on a medium proportion of VA (approximately 4 to 30%), is referred to as thermoplastic ethylene-vinyl acetate copolymer and is a thermoplastic elastomer material. It is not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrylonitrile Butadiene Styrene
Acrylonitrile butadiene styrene (ABS) (chemical formula (C8H8)''x''·(C4H6)''y''·(C3H3N)''z'' is a common thermoplastic polymer. Its glass transition temperature is approximately . ABS is amorphous and therefore has no true melting point. ABS is a terpolymer made by polymerizing styrene and acrylonitrile in the presence of polybutadiene. The proportions can vary from 15% to 35% acrylonitrile, 5% to 30% butadiene and 40% to 60% styrene. The result is a long chain of polybutadiene crisscrossed with shorter chains of poly(styrene-co-acrylonitrile). The nitrile groups from neighboring chains, being polar, attract each other and bind the chains together, making ABS stronger than pure polystyrene. The acrylonitrile also contributes chemical resistance, fatigue resistance, hardness, and rigidity, while increasing the heat deflection temperature. The styrene gives the plastic a shiny, impervious surface, as well as hardness, rigidity, and improved processing ease. The polybutadiene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Styrene-acrylonitrile
Styrene acrylonitrile resin is a copolymer plastic consisting of styrene and acrylonitrile. It is also known as SAN. It is widely used in place of polystyrene owing to its greater thermal resistance. The chains of between 70 and 80% by weight styrene and 20 to 30% acrylonitrile. Larger acrylonitrile content improves mechanical properties and chemical resistance, but also adds a yellow tint to the normally transparent plastic. Properties SAN is similar in use to polystyrene. Like polystyrene itself, it is optically transparent and brittle in mechanical behavior. The copolymer has a glass transition temperature greater than 100 °C owing to the acrylonitrile units in the chain, thus making the material resistant to boiling water. It is structurally related to ABS plastic, where polybutadiene is copolymerised with SAN to give a much tougher material. The rubber chains form separate phases which are 10-20 micrometers in diameter. When the product is stressed, crazing from the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization. Classification Monomers can be classified in many ways. They can be subdivided into two broad classes, depending on the kind of the polymer that they form. Monomers that participate in condensation polymerization have a different stoichiometry than monomers that participate in addition polymerization: : Other classifications include: *natural vs synthetic monomers, e.g. glycine vs caprolactam, respectively *polar vs nonpolar monomers, e.g. vinyl acetate vs ethylene, respectively *cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively The polymerization of one kind of monomer gives a homopolymer. Many polymers are copolymers, meaning that they are derived from two different monomers. In the case of condensation polymerizations, the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Styrene/butadiene Co-polymer
Styrene-butadiene or styrene-butadiene rubber (SBR) describe families of synthetic rubbers derived from styrene and butadiene (the version developed by Goodyear is called Neolite). These materials have good abrasion resistance and good aging stability when protected by additives. In 2012, more than 5.4 million tonnes of SBR were processed worldwide. About 50% of car tires are made from various types of SBR. The styrene/butadiene ratio influences the properties of the polymer: with high styrene content, the rubbers are harder and less rubbery. SBR is not to be confused with the thermoplastic elastomer, styrene-butadiene block copolymer, although being derived from the same monomers. Types of SBR SBR is derived from two monomers, styrene and butadiene. The mixture of these two monomers is polymerized by two processes: from solution (S-SBR) or as an emulsion (E-SBR). E-SBR is more widely used. Emulsion polymerization E-SBR produced by emulsion polymerization is initiated by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chain-growth Polymerization
Chain-growth polymerization (American English, AE) or chain-growth polymerisation (British English, BE) is a polymerization technique where Unsaturated compound, unsaturated monomer molecules add onto the active site on a growing polymer chain one at a time. There are a limited number of these active sites at any moment during the polymerization which gives this method its key characteristics. Introduction In 1953, Paul Flory first classified polymerization as "step-growth polymerization" and "chain-growth polymerization". IUPAC recommends to further simplify "chain-growth polymerization" to "chain polymerization". It is a kind of polymerization where an active center (free radical or ion) is formed, and a plurality of monomers can be polymerized together in a short period of time to form a macromolecule having a large molecular weight. In addition to the regenerated active sites of each monomer unit, polymer growth will only occur at one (or possibly more) endpoint. Many comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer Chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures of chemicals, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applicable through a wide range of other chemistry sub-disciplines like organic chemistry, analytical chemistry, and physical chemistry. Many materials have polymeric structures, from fully inorganic metals and ceramics to DNA and other biological molecules. However, polymer chemistry is typically related to synthetic and organic compositions. Synthetic polymers are ubiquitous in commercial materials and products in everyday use, such as plastics, and rubbers, and are major components of composite materials. Polymer chemistry can also be included in the broader fields of polymer science or even nanotechnology, both of which can be described as encompassing polymer physics and polymer engineering.Hans-Heinrich Moretto, Manfred Sch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |