|
Cobalt 60
Cobalt-60 (60Co) is a synthetic radioactive isotope of cobalt with a half-life of 5.2713 years. It is produced artificially in nuclear reactors. Deliberate industrial production depends on neutron activation of bulk samples of the monoisotopic and mononuclidic cobalt isotope . (PDF also located aCanadian Nuclear FAQ Measurable quantities are also produced as a by-product of typical nuclear power plant operation and may be detected externally when leaks occur. In the latter case (in the absence of added cobalt) the incidentally produced is largely the result of multiple stages of neutron activation of iron isotopes in the reactor's steel structures via the creation of its precursor. The simplest case of the latter would result from the activation of . undergoes beta decay to the stable isotope nickel-60 (). The activated nickel nucleus emits two gamma rays with energies of 1.17 and 1.33 MeV, hence the overall equation of the nuclear reaction (activation and decay) is: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace Radioisotope
A trace radioisotope is a radioisotope that occurs naturally in trace amounts (i.e. extremely small). Generally speaking, trace radioisotopes have half-lives that are short in comparison with the age of the Earth, since primordial nuclides tend to occur in larger than trace amounts. Trace radioisotopes are therefore present only because they are continually produced on Earth by natural processes. Natural processes which produce trace radioisotopes include cosmic ray bombardment of stable nuclides, ordinary alpha and beta decay of the long-lived heavy nuclides, thorium-232, uranium-238, and uranium-235, spontaneous fission of uranium-238, and nuclear transmutation reactions induced by natural radioactivity, such as the production of plutonium-239 and uranium-236 from neutron capture by natural uranium. Elements The elements that occur on Earth only in traces are listed below. Isotopes of other elements (not exhaustive): *Tritium * Beryllium-7 *Beryllium-10 *Carbon-14 *Fluorin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
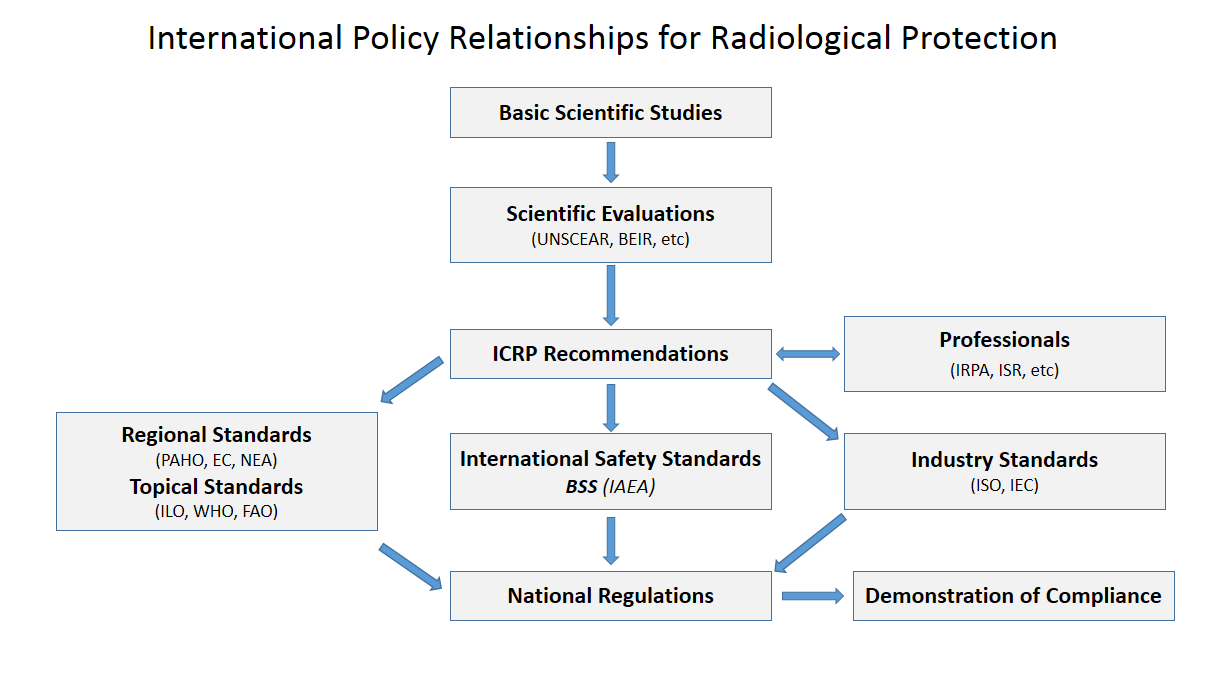
International Atomic Energy Agency
The International Atomic Energy Agency (IAEA) is an intergovernmental organization that seeks to promote the peaceful use of nuclear energy and to inhibit its use for any military purpose, including nuclear weapons. It was established in 1957 as an autonomous organization within the United Nations system; though governed by its own founding treaty, the organization reports to both the General Assembly and the Security Council of the United Nations, and is headquartered at the UN Office at Vienna, Austria. The IAEA was created in response to growing international concern toward nuclear weapons, especially amid rising tensions between the foremost nuclear powers, the United States and the Soviet Union. U.S. President Dwight D. Eisenhower's " Atoms for Peace" speech, which called for the creation of an international organization to monitor the global proliferation of nuclear resources and technology, is credited with catalyzing the formation of the IAEA, whose treaty came into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Scheme
The decay scheme of a radioactive substance is a graphical presentation of all the transitions occurring in a decay, and of their relationships. Examples are shown below. It is useful to think of the decay scheme as placed in a coordinate system, where the vertical axis is energy, increasing from bottom to top, and the horizontal axis is the proton number, increasing from left to right. The arrows indicate the emitted particles. For the gamma rays (vertical arrows), the gamma energies are given; for the beta decay (oblique arrow), the maximum beta energy. Examples These relations can be quite complicated; a simple case is shown here: the decay scheme of the radioactive cobalt isotope cobalt-60.K.H.Lieser ''Einführung in die Kernchemie'' (1991) S.223, Abb. (7-22); 60Co decays by emitting an electron (beta decay) with a half-life of 5.272 years into an excited state of 60Ni, which then decays very fast to the ground state of 60Ni, via two gamma decays. All known decay schemes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Mass Unit
The dalton or unified atomic mass unit (symbols: Da or u) is a non-SI unit of mass widely used in physics and chemistry. It is defined as of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at rest. The atomic mass constant, denoted ''m''u, is defined identically, giving . This unit is commonly used in physics and chemistry to express the mass of atomic-scale objects, such as atoms, molecules, and elementary particles, both for discrete instances and multiple types of ensemble averages. For example, an atom of helium-4 has a mass of . This is an intrinsic property of the isotope and all helium-4 atoms have the same mass. Acetylsalicylic acid (aspirin), , has an average mass of approximately . However, there are no acetylsalicylic acid molecules with this mass. The two most common masses of individual acetylsalicylic acid molecules are , having the most common isotopes, and , in which one carbon is carbon-13. The molecular mass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equivalent Dose
Equivalent dose is a dose quantity '' H '' representing the stochastic health effects of low levels of ionizing radiation on the human body which represents the probability of radiation-induced cancer and genetic damage. It is derived from the physical quantity absorbed dose, but also takes into account the biological effectiveness of the radiation, which is dependent on the radiation type and energy. In the SI system of units, the unit of measure is the sievert (Sv). Application To enable consideration of stochastic health risk, calculations are performed to convert the physical quantity absorbed dose into equivalent dose, the details of which depend on the radiation type. For applications in radiation protection and dosimetry assessment, the International Commission on Radiological Protection (ICRP) and the International Commission on Radiation Units and Measurements (ICRU) have published recommendations and data on how to calculate equivalent dose from absorbed dose. Equiv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sievert
The sievert (symbol: SvNot be confused with the sverdrup or the svedberg, two non-SI units that sometimes use the same symbol.) is a unit in the International System of Units (SI) intended to represent the stochastic health risk of ionizing radiation, which is defined as the probability of causing radiation-induced cancer and genetic damage. The sievert is important in dosimetry and radiation protection. It is named after Rolf Maximilian Sievert, a Swedish medical physicist renowned for work on radiation dose measurement and research into the biological effects of radiation. The sievert is used for radiation dose quantities such as equivalent dose and effective dose (radiation), effective dose, which represent the risk of external radiation from sources outside the body, and committed dose, which represents the risk of internal irradiation due to inhaled or ingested radioactive substances. According to the International Commission on Radiological Protection (ICRP) one sievert r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram
The gram (originally gramme; SI unit symbol g) is a Physical unit, unit of mass in the International System of Units (SI) equal to one one thousandth of a kilogram. Originally defined as of 1795 as "the absolute weight of a volume of pure water equal to Cube (algebra), the cube of the hundredth part of a metre [1 Cubic centimetre, cm3], and at Melting point of water, the temperature of Melting point, melting ice", the defining temperature (~0 °C) was later changed to 4 °C, the temperature of maximum density of water. However, by the late 19th century, there was an effort to make the Base unit (measurement), base unit the kilogram and the gram a derived unit. In 1960, the new International System of Units defined a ''gram'' as one one-thousandth of a kilogram (i.e., one gram is Scientific notation, 1×10−3 kg). The kilogram, 2019 redefinition of the SI base units, as of 2019, is defined by the International Bureau of Weights and Measures from the fixed numeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive Decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha decay ( ), beta decay ( ), and gamma decay ( ), all of which involve emitting one or more particles. The weak force is the mechanism that is responsible for beta decay, while the other two are governed by the electromagnetism and nuclear force. A fourth type of common decay is electron capture, in which an unstable nucleus captures an inner electron from one of the electron shells. The loss of that electron from the shell results in a cascade of electrons dropping down to that lower shell resulting in emission of discrete X-rays from the transitions. A common example is iodine-125 commonly used in medical settings. Radioactive decay is a stochastic (i.e. random) proce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Megaelectronvolt
In physics, an electronvolt (symbol eV, also written electron-volt and electron volt) is the measure of an amount of kinetic energy gained by a single electron accelerating from rest through an electric potential difference of one volt in vacuum. When used as a unit of energy, the numerical value of 1 eV in joules (symbol J) is equivalent to the numerical value of the charge of an electron in coulombs (symbol C). Under the 2019 redefinition of the SI base units, this sets 1 eV equal to the exact value Historically, the electronvolt was devised as a standard unit of measure through its usefulness in electrostatic particle accelerator sciences, because a particle with electric charge ''q'' gains an energy after passing through a voltage of ''V.'' Since ''q'' must be an integer multiple of the elementary charge ''e'' for any isolated particle, the gained energy in units of electronvolts conveniently equals that integer times the voltage. It is a common unit of energy within p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma Ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (), it imparts the highest photon energy. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation ''gamma rays'' based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power. Gamma rays from radioactive decay are in the energy range from a few kiloelectronvolts (keV) to approximately 8 megaelectronvolts (MeV), corresponding to the typical energy levels in nuclei with reasonably long lif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)
