|
Clopamide
Clopamide (trade name Brinaldix) is a piperidine diuretic. Mechanism of action Clopamide is categorised as a thiazide-like diuretic and works in similar way as the thiazide diuretics do. It acts in the kidneys, at the distal convoluted tubule (DCT) of the nephron where it inhibits the sodium-chloride symporter. Clopamide selectively binds at the chloride binding site of the sodium-chloride symporter in the PCT cells on the luminal (interior) side and thus interferes with the reabsorption of sodium chloride, causing an equiosmolar Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution (osmol/L or Osm/L). The osmolarity of a solution is usually expressed as Osm/L ... excretion of water along with sodium chloride. References {{Diuretics Piperidines Sulfonamides Hydrazides Novartis brands Diuretics Carbonic anhydrase inhibitors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperidine
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic compound, heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, and typical of amines. The name comes from the genus name ''Piper (genus), Piper'', which is the Latin word for Black pepper, pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring Solenopsin, solenopsins. Production Piperidine was first reported in 1850 by the Scottish chemist Thomas Anderson (chemist), Thomas Anderson and again, independently, in 1852 by the French chemist Auguste André Thomas Cahours, Auguste Cahours, who named it. Both of them obtained piperidine by reacting piperine with nitric acid. Industrially, piperidine is produced by the hydrogenation o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diuretic
A diuretic () is any substance that promotes diuresis, the increased production of urine. This includes forced diuresis. A diuretic tablet is sometimes colloquially called a water tablet. There are several categories of diuretics. All diuretics increase the excretion of water from the body, through the kidneys. There exist several classes of diuretic, and each works in a distinct way. Alternatively, an antidiuretic, such as vasopressin ( antidiuretic hormone), is an agent or drug which reduces the excretion of water in urine. Medical uses In medicine, diuretics are used to treat heart failure, liver cirrhosis, hypertension, influenza, water poisoning, and certain kidney diseases. Some diuretics, such as acetazolamide, help to make the urine more alkaline, and are helpful in increasing excretion of substances such as aspirin in cases of overdose or poisoning. Diuretics are sometimes abused by people with an eating disorder, especially people with bulimia nervosa, with the goa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiazide-like Diuretic
A thiazide-like diuretic is a sulfonamide diuretic that has similar physiological properties to a thiazide diuretic, but does not have the chemical properties of a thiazide, lacking the benzothiadiazine molecular structure. Examples include metolazone, chlorthalidone, and indapamide Indapamide is a thiazide-like diuretic drug used in the treatment of hypertension, as well as decompensated heart failure. Combination preparations with perindopril (an ACE inhibitor antihypertensive) are available. The thiazide-like diuretics ( .... References Diuretics {{Cardiovascular-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiazide
Thiazide () refers to both a class of sulfur-containing organic molecules and a class of diuretics based on the chemical structure of benzothiadiazine. The thiazide drug class was discovered and developed at Merck and Co. in the 1950s. The first approved drug of this class, chlorothiazide, was marketed under the trade name Diuril beginning in 1958. In most countries, thiazides are the least expensive antihypertensive drugs available. Thiazide organic molecules are bi-cyclic structures that contain adjacent sulfur and nitrogen atoms on one ring. Confusion sometimes occurs because thiazide-like diuretics such as indapamide are referred to as thiazides despite not having the thiazide chemical structure. When used this way, "thiazide" refers to a drug which acts at the thiazide receptor. The thiazide receptor is a sodium-chloride transporter that pulls NaCl from the lumen in the distal convoluted tubule. Thiazide diuretics inhibit this receptor, causing the body to release NaCl and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distal Convoluted Tubule
The distal convoluted tubule (DCT) is a portion of kidney nephron between the loop of Henle and the collecting tubule. Physiology It is partly responsible for the regulation of potassium, sodium, calcium, and pH. On its apical surface (lumen side), cells of the DCT have a thiazide-sensitive Na-Cl cotransporter and are permeable to Ca, via the TRPV5 channel. On the basolateral surface (peritubular capillary side) there is an ATP-dependent Na/K antiporter pump, a secondary active Na/Ca transporter, and an ATP dependent Ca transporter. The basolateral ATP dependent Na/K pump produces the gradient for Na to be absorbed from the apical surface via the Na/Cl symporter, and for Ca to be reclaimed into the blood by the Na/Ca basolateral antiporter. * It regulates pH by absorbing bicarbonate and secreting protons (H+) into the filtrate, or by absorbing protons and secreting bicarbonate into the filtrate. * Sodium and potassium levels are controlled by secreting K+ and absorbing Na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nephron
The nephron is the minute or microscopic structural and functional unit of the kidney. It is composed of a renal corpuscle and a renal tubule. The renal corpuscle consists of a tuft of capillaries called a glomerulus and a cup-shaped structure called Bowman's capsule. The renal tubule extends from the capsule. The capsule and tubule are connected and are composed of epithelial cells with a lumen. A healthy adult has 1 to 1.5 million nephrons in each kidney. Blood is filtered as it passes through three layers: the endothelial cells of the capillary wall, its basement membrane, and between the foot processes of the podocytes of the lining of the capsule. The tubule has adjacent peritubular capillaries that run between the descending and ascending portions of the tubule. As the fluid from the capsule flows down into the tubule, it is processed by the epithelial cells lining the tubule: water is reabsorbed and substances are exchanged (some are added, others are removed); first with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium-chloride Symporter
The sodium-chloride symporter (also known as Na+-Cl− cotransporter, NCC or NCCT, or as the thiazide-sensitive Na+-Cl− cotransporter or TSC) is a cotransporter in the kidney which has the function of reabsorbing sodium and chloride ions from the tubular fluid into the cells of the distal convoluted tubule of the nephron. It is a member of the SLC12 cotransporter family of electroneutral cation-coupled chloride cotransporters. In humans, it is encoded by the gene (solute carrier family 12 member 3) located in 16q13. A loss of NCC function causes Gitelman syndrome, an autosomic recessive disease characterized by salt wasting and low blood pressure, hypokalemic metabolic alkalosis, hypomagnesemia and hypocalciuria. Over a hundred different mutations in the NCC gene have been identified. Molecular biology The sodium-chloride symporter or NCC is a member of the SLC12 cotransporter family of electroneutral cation-coupled chloride cotransporter, along with the potassium-chlori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, salt (also known as ''table salt'') is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is de-icing of roadways in sub-freezing weather. Uses In addition to the familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year production (2008 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmolar
Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution (osmol/L or Osm/L). The osmolarity of a solution is usually expressed as Osm/L (pronounced "osmolar"), in the same way that the molarity of a solution is expressed as "M" (pronounced "molar"). Whereas molarity measures the number of moles of solute per unit volume of solution, osmolarity measures the number of ''osmoles of solute particles'' per unit volume of solution. This value allows the measurement of the osmotic pressure of a solution and the determination of how the solvent will diffuse across a semipermeable membrane (osmosis) separating two solutions of different osmotic concentration. Unit The unit of osmotic concentration is the osmole. This is a non- SI unit of measurement that defines the number of moles of solute that contribute to the osmotic pressure of a solution. A milliosmole (mOsm) is 1/1,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperidines
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, and typical of amines. The name comes from the genus name ''Piper'', which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins. Production Piperidine was first reported in 1850 by the Scottish chemist Thomas Anderson and again, independently, in 1852 by the French chemist Auguste Cahours, who named it. Both of them obtained piperidine by reacting piperine with nitric acid. Industrially, piperidine is produced by the hydrogenation of pyridine, usually over a molybdenum disulfide catalyst: : C5H5N + 3 H2 → C5H10NH Pyridine can also be reduce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
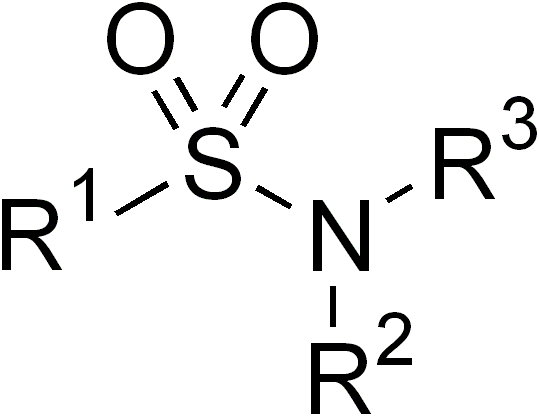
Sulfonamides
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |