|
Clinicaltrials.gov
ClinicalTrials.gov is a registry of clinical trials. It is run by the United States National Library of Medicine (NLM) at the National Institutes of Health, and is the largest clinical trials database, holding registrations from over 329,000 trials from 209 countries. History As a result of pressure from HIV-infected men in the gay community, who demanded better access to clinical trials, the U.S. Congress passed the Health Omnibus Programs Extension Act of 1988 (Public Law 100-607) which mandated the development of a database of AIDS Clinical Trials Information Services (ACTIS). This effort served as an example of what might be done to improve public access to clinical trials, and motivated other disease-related interest groups to push for something similar for all diseases. The Food and Drug Administration Modernization Act of 1997 (Public Act 105-115) amended the Food, Drug and Cosmetic Act and the Public Health Service Act to require that the NIH create and operate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
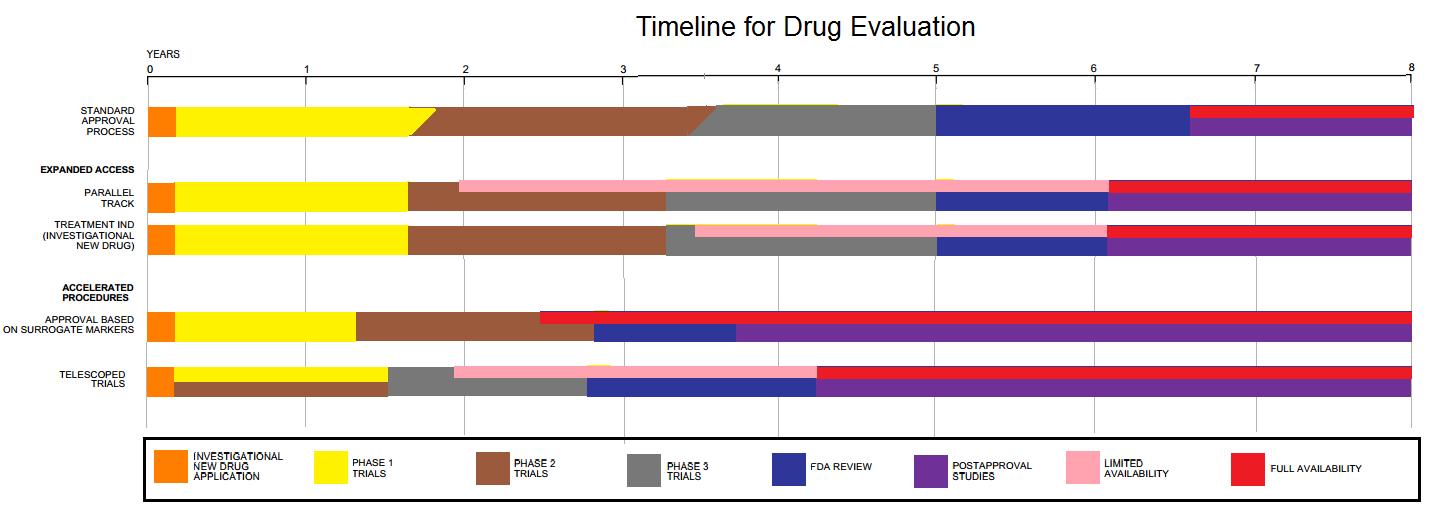
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can vary i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration Amendments Act Of 2007
President of the United States George W. Bush signed the Food and Drug Administration Amendments Act of 2007 (FDAAA) on September 27, 2007. This law reviewed, expanded, and reaffirmed several existing pieces of legislation regulating the FDA. These changes allow the FDA to perform more comprehensive reviews of potential new drugs and devices. It was sponsored by Reps. Joe Barton and Frank Pallone and passed unanimously by the Senate. The FDAAA extended the authority to levy fees to companies applying for approval of drugs, expanded clinical trial guidelines for pediatric drugs, and created the priority review voucher program, amongst other items. Title I: Prescription Drug User Fee Amendments of 2007 Title I amends the Federal Food, Drug, and Cosmetic Act to include post-marketing safety activities in the review of drug application. This included developing and using improved adverse event data collection systems and improved analytical tools to assess potential safety probl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adverse Events
An adverse event (AE) is any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment. An adverse event can therefore be any unfavourable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medicinal (investigational) product, whether or not related to the medicinal (investigational) product. AEs in patients participating in clinical trials must be reported to the study sponsor and if required could be reported to the local ethics committee. Adverse events categorized as "serious" (results in death, illness requiring hospitalization, events deemed life-threatening, results in persistent or significant incapacity, a congenital anomaly, birth defect or medically important condition) must be reported to the regulatory authorities immediately, whereas non-serious adverse events are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trials Registry
Preregistration is the practice of registering the hypotheses, methods, and/or analyses of a scientific study before it is conducted. This can include analyzing primary data or secondary data. Clinical trial registration is similar, although it may not require the registration of a study's analysis protocol. Finally, registered reports include the peer review and in principle acceptance of a study protocol prior to data collection. Preregistration assists in the identification and/or reduction of a variety of potentially problematic research practices, including p-hacking, publication bias, data dredging, inappropriate forms of post hoc analysis, and (relatedly) HARKing. It has recently gained prominence in the open science community as a potential solution to some of the issues that are thought to underlie the replication crisis. However, critics have argued that it may not be necessary when other open science practices are implemented such as Registered Reports. Types Standa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United States National Library Of Medicine
The United States National Library of Medicine (NLM), operated by the United States federal government, is the world's largest medical library. Located in Bethesda, Maryland, the NLM is an institute within the National Institutes of Health. Its collections include more than seven million books, journals, technical reports, manuscripts, microfilms, photographs, and images on medicine and related sciences, including some of the world's oldest and rarest works. The current director of the NLM is Patricia Flatley Brennan.National Library of Medicine Welcomes New Director Dr. Patricia Flatley Brennan . ''National Library of Medicine''. August 15, 2016. History The precursor o ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trials Registry
Preregistration is the practice of registering the hypotheses, methods, and/or analyses of a scientific study before it is conducted. This can include analyzing primary data or secondary data. Clinical trial registration is similar, although it may not require the registration of a study's analysis protocol. Finally, registered reports include the peer review and in principle acceptance of a study protocol prior to data collection. Preregistration assists in the identification and/or reduction of a variety of potentially problematic research practices, including p-hacking, publication bias, data dredging, inappropriate forms of post hoc analysis, and (relatedly) HARKing. It has recently gained prominence in the open science community as a potential solution to some of the issues that are thought to underlie the replication crisis. However, critics have argued that it may not be necessary when other open science practices are implemented such as Registered Reports. Types Standa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Library Of Medicine
The United States National Library of Medicine (NLM), operated by the United States federal government, is the world's largest medical library. Located in Bethesda, Maryland, the NLM is an institute within the National Institutes of Health. Its collections include more than seven million books, journals, technical reports, manuscripts, microfilms, photographs, and images on medicine and related sciences, including some of the world's oldest and rarest works. The current director of the NLM is Patricia Flatley Brennan.National Library of Medicine Welcomes New Director Dr. Patricia Flatley Brennan . ''National Library of Medicine''. August 15, 2016. History The precursor o ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Contract Research Organization
In the life sciences, a contract research organization (CRO) is a company that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. A CRO may provide such services as biopharmaceutical development, biological assay development, commercialization, clinical development, clinical trials management, pharmacovigilance, outcomes research, and Real world evidence. CROs are designed to reduce costs for companies developing new medicines and drugs in niche markets. They aim to simplify entry into drug markets, and simplify development, as the need for large pharmaceutical companies to do everything ‘in house’ is now redundant. CROs also support foundations, research institutions, and universities, in addition to governmental organizations (such as the NIH, EMA, etc.). Many CROs specifically provide clinical-study and clinical-trial support for drugs and/or medical devices. However, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process – from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials – to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Recall
A drug recall removes a prescription or over-the-counter drug from the market. Drug recalls in the United States are made by the FDA or the creators of the drug when certain criteria are met. When a drug recall is made, the drug is removed from the market and potential legal action can be taken depending on the severity of the drug recall. Drug recalls are classified in the US by the FDA in three different categories. Class I recalls are the most severe and indicate that exposure and/or consumption of the drug will lead to adverse health effects or death. Class II recalls refer to drugs that induce temporary and/or medically reversible health effects. Class III recalls occur when adverse health effects are not likely to occur when consuming the drug or being exposed to it. There are also market withdrawals and medical device safety alerts'. Market withdrawals occur when a product has a minor violation that does not require FDA legal action. Medical device safety alerts occur whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bethesda, Maryland
Bethesda () is an unincorporated, census-designated place in southern Montgomery County, Maryland. It is located just northwest of Washington, D.C. It takes its name from a local church, the Bethesda Meeting House (1820, rebuilt 1849), which in turn took its name from Jerusalem's Pool of Bethesda. The National Institutes of Health's main campus and the Walter Reed National Military Medical Center are in Bethesda, in addition to a number of corporate and government headquarters. As an unincorporated community, Bethesda has no official boundaries. According to the 2020 U.S. census, the community had a total population of 68,056. History Bethesda is located in a region that was populated by the Piscataway and Nacotchtank tribes at the time of European colonization. Fur trader Henry Fleet became the first European to visit the area, reaching it by sailing up the Potomac River. He stayed with the Piscataway tribe from 1623 to 1627, either as a guest or prisoner (historical accounts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)