|
Clinical Endpoint
Clinical endpoints or clinical outcomes are Outcome measure, outcome measures referring to occurrence of disease, symptom, Medical sign, sign or laboratory abnormality constituting a target outcome in clinical trial, clinical research trials. The term may also refer to any disease or sign that strongly motivates withdrawal of an individual or entity from the trial, then often termed a ''humane (clinical) endpoint''. The primary endpoint of a clinical trial is the endpoint for which the trial is statistical power, powered. Secondary endpoints are additional endpoints, preferably also pre-specified, for which the trial may not be powered. Surrogate endpoint, Surrogate endpoints are trial endpoints that have outcomes that substitute for a clinical endpoint, often because studying the clinical endpoint is difficult, for example using an increase in blood pressure as a surrogate for death by cardiovascular disease, where strong evidence of a causal link exists. Scope In a general sens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Outcome Measure
An outcome measure, endpoint, effect measure or measure of effect is a measure within medical practice or research, (primarily clinical trials) which is used to assess the effect, both positive and negative, of an intervention or treatment. Measures can often be quantified using effect sizes. Outcomes measures can be Patient-reported outcome, patient-reported, or gathered through laboratory tests such as Blood test, blood work, Clinical urine tests, urine samples etc. or through Physical examination, medical examination. Outcomes measures should be relevant to the target of the intervention (be it a single person or a target population). Depending on the design of a trial, outcome measures can be either primary outcomes, in which case the trial is designed around finding an adequate study size (through proper Random assignment, randomization and Statistical power, power calculation). Secondary or tertiary outcomes are outcome measures which are added after the design of the study is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
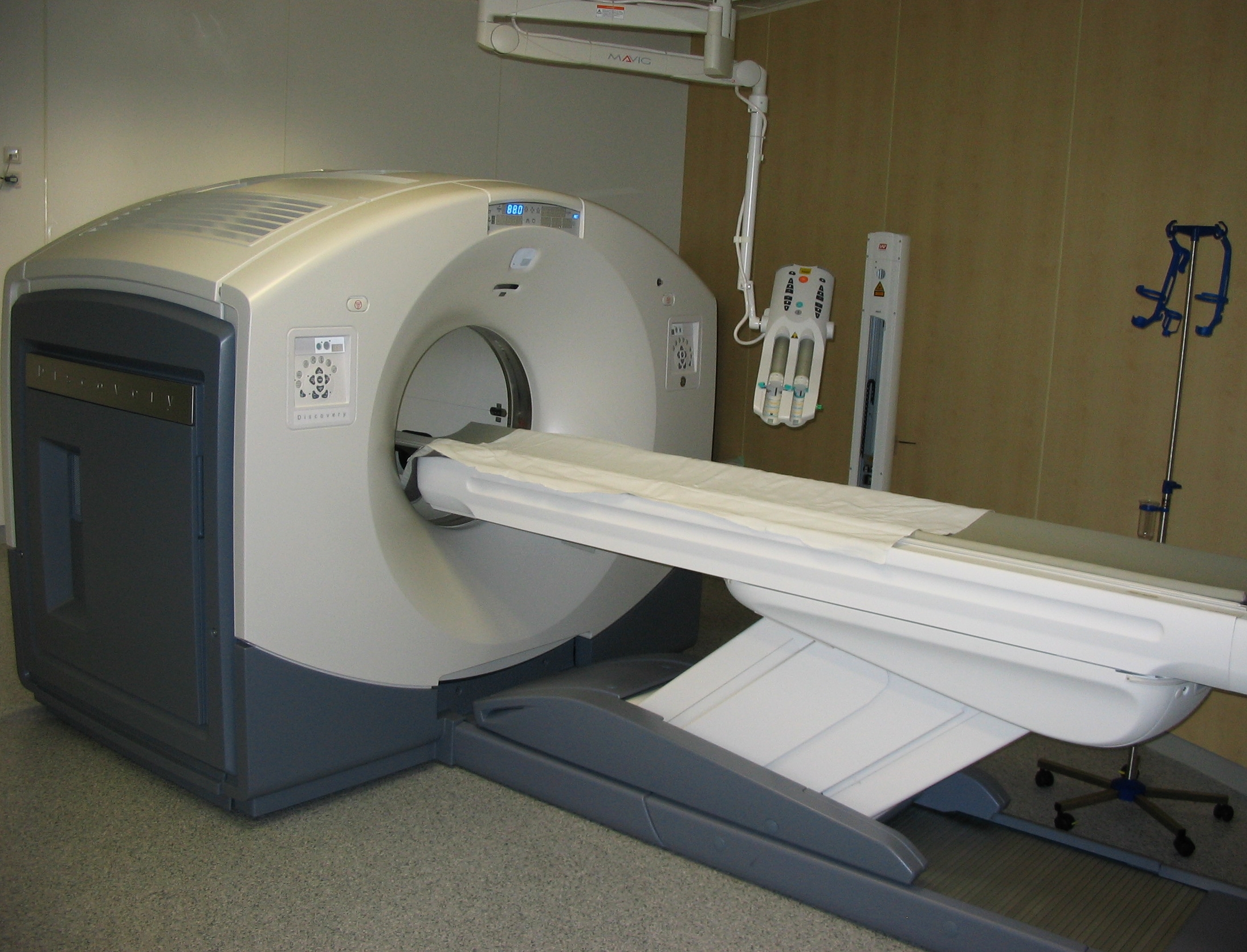
Positron Emission Tomography
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in Metabolism, metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body. For example, 18F-FDG, -FDG is commonly used to detect cancer, Sodium fluoride#Medical imaging, NaF is widely used for detecting bone formation, and Isotopes of oxygen#Oxygen-15, oxygen-15 is sometimes used to measure blood flow. PET is a common medical imaging, imaging technique, a Scintigraphy#Process, medical scintillography technique used in nuclear medicine. A radiopharmaceutical, radiopharmaceutical — a radioisotope attached to a drug — is injected into the body as a radioactive tracer, tracer. When the radiopharmaceutical undergoes beta plus decay, a positron is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Research
Clinical research is a branch of healthcare science that determines the safety and effectiveness ( efficacy) of medications, devices, diagnostic products and treatment regimens intended for human use. These may be used for prevention, treatment, diagnosis or for relieving symptoms of a disease. Clinical research is different from clinical practice. In clinical practice established treatments are used, while in clinical research evidence is collected to establish a treatment. Overview The term "clinical research" refers to the entire bibliography of a drug/device/biologic, in fact any test article from its inception in the lab to its introduction to the consumer market and beyond. Once the promising candidate or the molecule is identified in the lab, it is subjected to pre-clinical studies or animal studies where different aspects of the test article (including its safety toxicity if applicable and efficacy, if possible at this early stage) are studied. In the United States ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immune-related Response Criteria
The immune-related response criteria (irRC) is a set of published rules that define when tumors in cancer patients improve ("respond"), stay the same ("stabilize"), or worsen ("progress") during treatment, where the compound being evaluated is an immuno-oncology drug. Immuno-oncology, part of the broader field of cancer immunotherapy, involves agents which harness the body's own immune system to fight cancer. Traditionally, patient responses to new cancer treatments have been evaluated using two sets of criteria, the WHO criteria and the response evaluation criteria in solid tumors (RECIST). The immune-related response criteria, first published in 2009, arose out of observations that immuno-oncology drugs would fail in clinical trials that measured responses using the WHO or RECIST Criteria, because these criteria could not account for the time gap in many patients between initial treatment and the apparent action of the immune system to reduce the tumor burden. Background Part o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |