|
Class A Drug
These drugs are known in the UK as ''controlled drugs'', because this is the term by which the act itself refers to them. In more general terms, however, many of these drugs are also controlled by the Medicines Act 1968, there are many other drugs which are controlled by the Medicines Act but not by the Misuse of Drugs Act, and some other drugs (alcohol, for example) are controlled by other laws. The Misuse of Drugs Act sets out three separate categories, Class A, Class B, and Class C. Class A drugs represent those deemed most dangerous, and so carry the harshest punishments. Class C represents those thought to have the least capacity for harm, and so the Act demands more lenient punishment. In reality the potential harm has little bearing on the class, which has led to dissatisfaction with drug laws. Being found in possession of a drug on this list is dealt with less seriously than would be if it were deemed that there is intent to supply (even without payment) the drug to o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medicines Act 1968
The Medicines Act 1968 is an Act of Parliament of the United Kingdom, more properly: An Act to make new provision with respect to medicinal products and related matters, and for purposes connected therewith. It governs the control of medicines for human use and for veterinary use, which includes the manufacture and supply of medicines, and the manufacture and supply of (medicated) animal feeding stuffs. The Act defines three categories of medicine: prescription only medicines (POM), which are available only from a pharmacist if prescribed by an appropriate practitioner (including, but not limited to doctors, dentists, optometrists and nurses); pharmacy medicines (P), available only from a pharmacist but without a prescription; and general sales list (GSL) medicines which may be bought from any shop without a prescription. The Act controls supply of the drugs it covers, but does not define any offence of simple possession. Possession of a prescription only drug without a prescri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
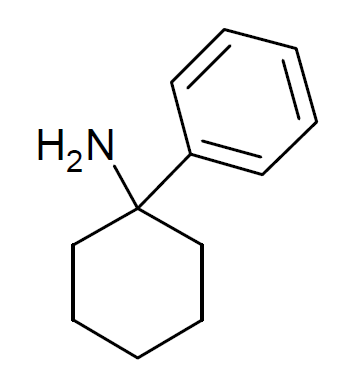
Arylcyclohexamine
Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs. History Phencyclidine (PCP) is believed to be the first arylcyclohexylamine with recognized anesthetic properties, but several arylcyclohexylamines were described before PCP in the scientific literature, beginning with PCA (1-phenylcyclohexan-1-amine) the synthesis of which was first published in 1907. PCE was reported in 1953 and PCMo (4-(1-phenyl-cyclohexyl)-morpholine see chart below for figure) in 1954, with PCMo described as a potent sedative. Arylcyclohexylamine anesthetics were intensively investigated at Parke-Davis, beginning with the 1956 synthesis of phencyclidine and later the related compound ketamine. The 1970s saw the debut of these compounds, especially PCP and its analogues, as illicitly used recreational drugs due to their dissociative hallucinogenic and euphoriant effects. Since that time, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anileridine
Anileridine (trade name: Leritine) is a synthetic analgesic drug and is a member of the piperidine class of analgesic agents developed by Merck & Co. in the 1950s. It differs from pethidine (meperidine) in that the ''N''-methyl group of meperidine is replaced by an ''N''-aminophenethyl group, which increases its analgesic activity. Anileridine is no longer manufactured in the US or Canada. Anileridine is in Schedule II of the Controlled Substances Act 1970 of the United States as ACSCN 9020 with a zero aggregate manufacturing quota as of 2014. The free base conversion ratio for salts includes 0.83 for the dihydrochloride and 0.73 for the phosphate. It is also under international control per UN treaties. Administration As tablets or injection. Pharmacokinetics Anileridine usually takes effect within 15 minutes of either oral or intravenous administration, and lasts 2–3 hours. It is mostly metabolized by the liver The liver is a major Organ (anatomy), organ only found ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alphaprodine
Prodine (trade names Prisilidine and Nisentil) is an opioid analgesic that is an analog of pethidine (meperidine). It was developed in Germany in the late 1940s. There are two isomers of the trans form of prodine, alphaprodine and betaprodine. Both exhibit optical isomerism and alphaprodine and betaprodine are racemates. Alphaprodine is closely related to desomorphine in steric configuration. The cis form also has active isomers but none are used in medicine. Betaprodine is around five times more potent than alphaprodine but is metabolized more rapidly, and only alphaprodine was developed for medicinal use. It has similar activity to pethidine, but with a more rapid onset and shorter duration of effects. Betaprodine produces more euphoria and side effects than alphaprodine at all dose levels, and it was found that 5 to 10 mg of betaprodine is equivalent to 25 to 40 mg of alphaprodine. Testing in rats showed alphaprodine to be 97% the strength of morphine via the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alphamethadol
Alphamethadol (INN), or α-methadol, also known as alfametadol, is a synthetic opioid analgesic. It is an isomer of dimepheptanol (methadol), the other being betamethadol (β-methadol). Alphamethadol is composed of two isomers itself, L-α-methadol, and D-α-methadol. The former compound, L-α-methadol, is an important active metabolite of levacetylmethadol (LAAM), an opioid substitute drug that is used clinically. Both of alphamethadol's isomers bind to and activate the μ-opioid receptor and are active as opioid analgesics, similarly to those of alphacetylmethadol (α-acetylmethadol). Legal status Australia Alphamethadol is considered a Schedule 9 prohibited substance in Australia under the Poisons Standard (February 2017).Poisons Standard October 2015 https://www.legislation.gov.au/Details/F2017L00057 A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alphameprodine
Meprodine is an opioid analgesic that is an analogue of pethidine (meperidine). It is closely related to the drug prodine, the only difference being that meprodine has an ethyl group rather than a methyl at the 3-position of the piperidine ring. As with prodine, there are two isomers of meprodine, alpha-meprodine and beta-meprodine, with the alpha isomer having been more widely used. Alphameprodine (ACSCN 9604) and betameprodine (ACSCN 9608) are both Schedule I Narcotic controlled substances in the United States, both with annual aggregate manufacturing quotas of 2 grammes as of 2014. Meprodine has similar effects to other opioids, and produces analgesia, sedation and euphoria. Side effects can include itching, nausea and potentially serious respiratory depression Hypoventilation (also known as respiratory depression) occurs when ventilation is inadequate (''hypo'' meaning "below") to perform needed respiratory gas exchange. By definition it causes an increased concentratio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alphacetylmethadol
Alphacetylmethadol (INN), or α-acetylmethadol (AAM), is a synthetic opioid analgesic. Its levorotary enantiomer, levacetylmethadol, is an FDA-approved treatment for opioid addiction. Alphacetylmethadol is very similar in structure to methadone, a widely prescribed treatment for opioid addiction. In the United States, it is a Schedule I controlled substance under the Controlled Substances Act (presumably because it was never marketed in the US, as is the case with other common opiate/opioid medications such as heroin and prodine), with an ACSCN of 9603 and a 2013 annual manufacturing quota of 2 grammes. See also * Levacetylmethadol * Acetylmethadol * Betacetylmethadol * Alphamethadol Alphamethadol ( INN), or α-methadol, also known as alfametadol, is a synthetic opioid analgesic. It is an isomer of dimepheptanol (methadol), the other being betamethadol (β-methadol). Alphamethadol is composed of two isomers itself, L-α-met ... References Acetate esters Dimeth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allylprodine
Allylprodine is an opioid analgesic that is an analog of prodine. It was discovered by Hoffman-La Roche in 1957 during research into the related drug pethidine. Derivatives were tested to prove the theory that phenolic & non-phenolic opioids bind at different sites of the opiate receptor. Allylprodine is more potent as an analgesic than similar drugs such as α-prodine, and the 3''R'',4''S''-isomer is 23 times more potent than morphine, due to the allyl group binding to an additional amino acid target in the binding site on the μ-opioid receptor. It is also stereoselective, with one isomer being much more active. When modeled in three dimensions, the alkene overlays the alkenes found in 14-cinnamoyloxycodeinone and in 14-allyloxycodeinone, re-enforcing the presence of an interaction of the alkene. Allylprodine produces similar effects to other opioids, such as analgesia and sedation, along with side effects such as nausea, itching, vomiting and respiratory depression which m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alfentanil
Alfentanil (R-39209, trade name Alfenta, Rapifen in Australia) is a potent but short-acting synthetic opioid analgesic medication, drug, used for anaesthesia in surgery. It is an analogue of fentanyl with around one-fourth to one-tenth the potency, one-third the duration of action, and an onset of action four times faster than that of fentanyl. Alfentanil has a pKa of approximately 6.5, which leads to a very high proportion of the drug being uncharged at physiologic pH, a characteristic responsible for its rapid onset. It is an agonist at mu opioid receptors. While alfentanil tends to cause fewer cardiovascular complications than other similar drugs such as fentanyl and remifentanil, it tends to give stronger respiratory depression and so requires careful monitoring of breathing and vital signs. Almost exclusively used by anesthesia providers during portions of a case where quick, fast-acting (though not long-lasting) pain control is needed (as, for example, during nerve blocks), a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetorphine
Acetorphine is a potent opioid analgesic, up to 8700 times stronger than morphine by weight. It is a derivative of the more well-known opioid etorphine, which is used as a very potent veterinary painkiller and anesthetic medication, primarily for the sedation of large animals such as elephants, giraffes and rhinos. Acetorphine was developed in 1966 by the Reckitt research group that developed etorphine. Acetorphine was developed for the same purpose as etorphine itself, namely as a strong tranquilizer for use in immobilizing large animals in veterinary medicine. Despite showing some advantages over etorphine, for instance producing less toxic side effects in giraffes, acetorphine was never widely adopted for veterinary use, and etorphine (along with other tranquilizers such as carfentanil and azaperone) remains the drug of choice in this application. Legal Status Australia Acetorphine is a schedule 9 substance in Australia under the Poisons Standard (February 2017). A schedule ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
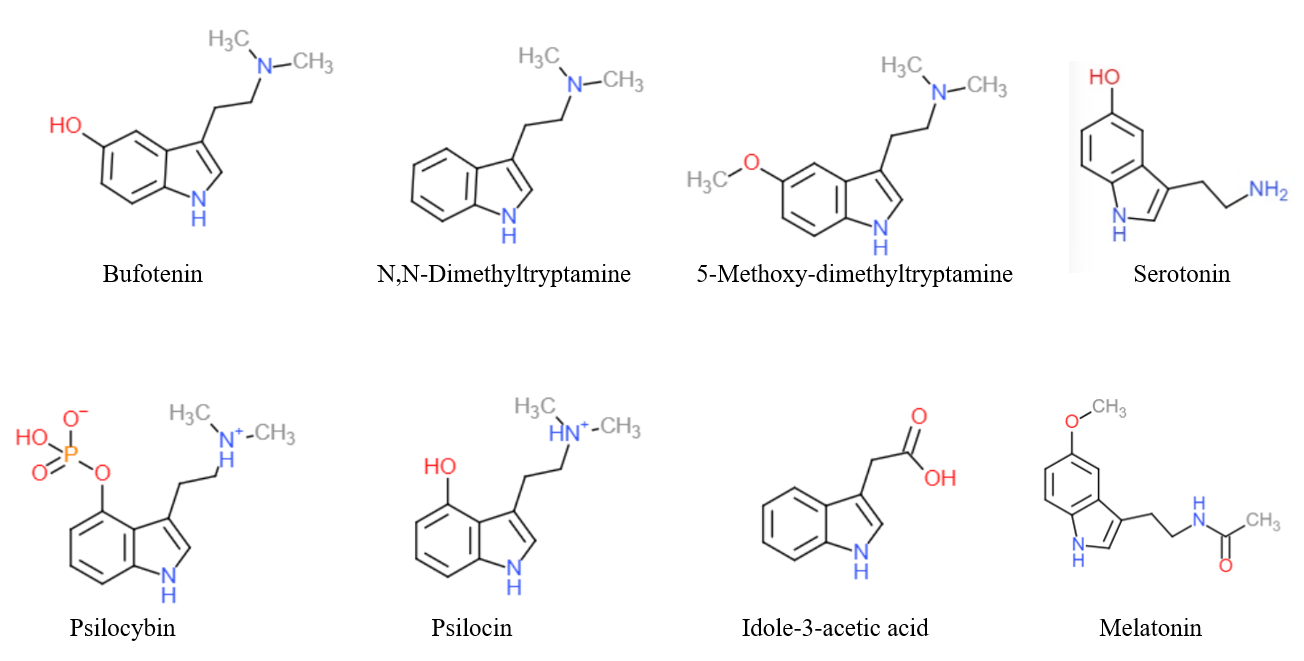
Tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stimulant
Stimulants (also often referred to as psychostimulants or colloquially as uppers) is an overarching term that covers many drugs including those that increase activity of the central nervous system and the body, drugs that are pleasurable and invigorating, or drugs that have Sympathomimetic drug, sympathomimetic effects. Stimulants are widely used throughout the world as prescription medicines as well as without a prescription (either legally or Prohibition (drugs), illicitly) as performance-enhancing substance, performance-enhancing or recreational drug use, recreational drugs. Among narcotics, stimulants produce a noticeable crash or ''Comedown (drugs), comedown'' at the end of their effects. The most frequently prescribed stimulants as of 2013 were lisdexamfetamine (Vyvanse), methylphenidate (Ritalin), and amphetamine (Adderall). It was estimated in 2015 that the percentage of the world population that had used cocaine during a year was 0.4%. For the category "amphetamines and p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |