|
Chloramine-T
Chloramine-T is the organic compound with the formula CH3C6H4SO2NClNa. Both the anhydrous salt and its trihydrate are known. Both are white powders. Chloramine-T is used as a reagent in organic synthesis. It is commonly used as cyclizing agent in the synthesis of aziridine, oxadiazole, isoxazole and pyrazoles. It's a inexpensive, low toxic and mild oxidizing agent, and it also acts as a source of nitrogen anions and eletrophilic cations. But it may undergo degradation on long term exposure to atmosphere, so care must be taken during the storage. Reactions Chloramine-T contains active ( electrophilic) chlorine. Its reactivity is similar to that of sodium hypochlorite. Aqueous solutions of chloramine-T are slightly basic ( pH typically 8.5). The p''K''a of the closely related ''N''-chlorophenylsulfonamide C6H5SO2NClH is 9.5. It is prepared by oxidation of toluenesulfonamide with sodium hypochlorite, with the latter being produced ''in situ'' from sodium hydroxide and chlorine ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfimide
In chemistry, a sulfilimine (or sulfimide) is a type of chemical compound containing a sulfur-to-nitrogen bond which is often represented as a double bond (). In fact, a double bond violates the octet rule, and the bond may be considered a single bond with a formal charge of +1 on the sulfur and a formal charge of −1 on the nitrogen. The parent compound is sulfilimine , which is mainly of theoretical interest. Examples include ''S'',''S''-diphenylsulfilimine and sulfoximines such as methylphenylsulfoximine: In the case of a sulfoximine, the bonds can be considered single bonds, with formal charges of −1 on both the oxygen and the nitrogen, and a formal charge of +2 on the sulfur. : Preparation Most sulfilimines are N-substituted with electron-withdrawing groups. These compounds are typically prepared by oxidation of thioethers with electrophilic amine reagents, such as chloramine-T in the presence of a base: :\mathbf\ce An alternative route involves reactions of electroph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sharpless Oxyamination
The Sharpless oxyamination (often known as Sharpless aminohydroxylation) is the chemical reaction that converts an alkene to a vicinal amino alcohol. The reaction is related to the Sharpless dihydroxylation, which converts alkenes to vicinal diols. Vicinal amino-alcohols are important products in organic synthesis and recurring pharmacophores in drug discovery. Mechanism Akin to the dihydroxylation, the oxyamination involves the cycloaddition of the alkene to an imido Os(VIII) intermediate of the type OsO3(NR). Such species are generated by treatment of osmium tetroxide with the sodium chloramines. Typical procedures combine chloramine-T, alkene, an osmium catalyst, and a chiral ligand. Related procedures use benzyl carbamate (CbzNH2), sodium hydroxide, tert-butyl hypochlorite to give CbzNCl(Na). :R2NH + t-BuOCl → R2NCl + t-BuOH Further reading Early papers in the development of this methodology. * Sharpless, K. B.; Patrick, D. W.; Truesdale, L. K.; Biller, S. A. ''J. Am. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability. Structure and characteristics of inorganic iodides Iodide is one of the largest monatomic anions. It is assigned a radius of around 206 picometers. For comparison, the lighter halides are considerably smaller: bromide (196 pm), chloride (181 pm), and fluoride (133 pm). In part because of its size, iodide forms relatively weak bonds with most elements. Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is not. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organochlorides
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine. The chloroalkane class (alkanes with one or more hydrogens substituted by chlorine) provides common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious. Physical and chemical properties Chlorination modifies the physical properties of hydrocarbons in several ways. These compounds are typically denser than water due to the higher atomic weight of chlorine versus hydrogen. Aliphatic organochlorides are often alkylating agents as chlorine can act as a leaving group, which can result in cellular damage. Natural occurrence Many organochlorine compounds have been isolate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
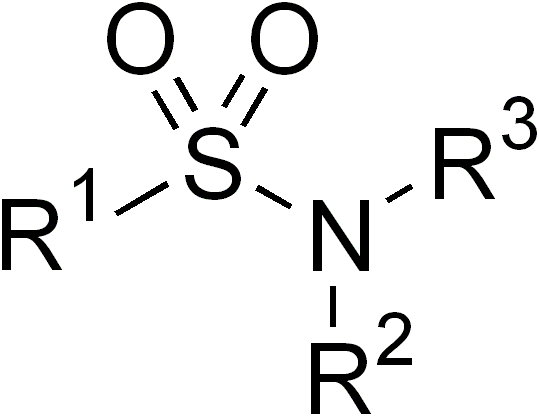
Sulfonamides
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pesticides
Pesticides are substances that are meant to control pests. This includes herbicide, insecticide, nematicide, molluscicide, piscicide, avicide, rodenticide, bactericide, insect repellent, animal repellent, microbicide, fungicide, and lampricide. The most common of these are herbicides which account for approximately 80% of all pesticide use. Most pesticides are intended to serve as plant protection products (also known as crop protection products), which in general, protect plants from weeds, fungi, or insects. As an example, the fungus ''Alternaria solani'' is used to combat the aquatic weed ''Salvinia''. In general, a pesticide is a chemical (such as carbamate) or biological agent (such as a virus, bacterium, or fungus) that deters, incapacitates, kills, or otherwise discourages pests. Target pests can include insects, plant pathogens, weeds, molluscs, birds, mammals, fish, nematodes (roundworms), and microbes that destroy property, cause nuisance, or spread disease, or ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiseptics
An antiseptic (from Greek ἀντί ''anti'', "against" and σηπτικός ''sēptikos'', "putrefactive") is an antimicrobial substance or compound that is applied to living tissue/skin to reduce the possibility of infection, sepsis, or putrefaction. Antiseptics are generally distinguished from ''antibiotics'' by the latter's ability to safely destroy bacteria within the body, and from ''disinfectants'', which destroy microorganisms found on non-living objects. Antibacterials include antiseptics that have the proven ability to act against bacteria. Microbicides which destroy virus particles are called viricides or antivirals. Antifungals, also known as antimycotics, are pharmaceutical fungicides used to treat and prevent mycosis (fungal infection). Surgery The widespread introduction of antiseptic surgical methods was initiated by the publishing of the paper ''Antiseptic Principle of the Practice of Surgery'' in 1867 by Joseph Lister, which was inspired by Louis Pasteur's ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clostridium Difficile (bacteria)
''Clostridioides difficile'' ( syn. ''Clostridium difficile'') is a bacterium that is well known for causing serious diarrheal infections, and may also cause colon cancer. Also known as ''C. difficile'', or ''C. diff'' (), is Gram-positive species of spore-forming bacteria. ''Clostridioides'' spp. are anaerobic, motile bacteria, ubiquitous in nature and especially prevalent in soil. Its vegetative cells are rod-shaped, pleomorphic, and occur in pairs or short chains. Under the microscope, they appear as long, irregular (often drumstick- or spindle-shaped) cells with a bulge at their terminal ends (forms subterminal spores). Under Gram staining, ''C. difficile'' cells are Gram-positive and show optimum growth on blood agar at human body temperatures in the absence of oxygen. ''C. difficile'' is catalase- and superoxide dismutase-negative, and produces up to three types of toxins: enterotoxin A, cytotoxin B and Clostridioides difficile transferase (CDT). Under stress condition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norovirus
Norovirus, sometimes referred to as the winter vomiting disease, is the most common cause of gastroenteritis. Infection is characterized by non-bloody diarrhea, vomiting, and stomach pain. Fever or headaches may also occur. Symptoms usually develop 12 to 48 hours after being exposed, and recovery typically occurs within one to three days. Complications are uncommon, but may include dehydration, especially in the young, the old, and those with other health problems. The virus is usually spread by the fecal–oral route. This may be through contaminated food or water or person-to-person contact. It may also spread via contaminated surfaces or through air from the vomit of an infected person. Risk factors include unsanitary food preparation and sharing close quarters. Diagnosis is generally based on symptoms. Confirmatory testing is not usually available but may be performed by public health agencies during outbreaks. Prevention involves proper hand washing and disinfection of con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of EN Standards
European Standards (abbreviated EN, from the German name ("European Norm")) are technical standards drafted and maintained by CEN (European Committee for Standardization), CENELEC (European Committee for Electrotechnical Standardization) and ETSI (European Telecommunications Standards Institute). EN 1–999 * EN 1: Flued oil stoves with vaporizing burners * EN 2: Classification of fires * EN 3: Portable fire extinguishers * EN 14: Dimensions of bed blankets * EN 19: Industrial valves – Marking of metallic valves *EN 20: Wood preservatives. * EN 26: Gas-fired instantaneous water heaters for the production of domestic hot water * EN 40-1: Lighting columns - Part 1: Definitions and terms * EN 40-2: Lighting columns - Part 2: General requirements and dimensions * EN 40-3-1: Lighting columns - Part 3-1: Design and verification - Specification for characteristic loads * EN 40-3-2: Lighting columns - Part 3-2: Design and verification - Verification by testing * EN 40-3-3: Light ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioiodine
There are 37 known isotopes of iodine (53I) from 108I to 144I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element. Its longest-lived radioactive isotope, 129I, has a half-life of 15.7 million years, which is far too short for it to exist as a primordial nuclide. Cosmogenic sources of 129I produce very tiny quantities of it that are too small to affect atomic weight measurements; iodine is thus also a mononuclidic element—one that is found in nature only as a single nuclide. Most 129I derived radioactivity on Earth is man-made, an unwanted long-lived byproduct of early nuclear tests and nuclear fission accidents. All other iodine radioisotopes have half-lives less than 60 days, and four of these are used as tracers and therapeutic agents in medicine. These are 123I, 124I, 125I, and 131I. All industrial production of radioactive iodine isotopes involves these four useful radionuclides. The isotope 135I has a half-life less than s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopic Labeling
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling. In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |