|
Caustic Ingestion
Caustic ingestion occurs when someone accidentally or deliberately ingests a corrosive substance, caustic or corrosive substance. Depending on the nature of the substance, the duration of exposure and other factors it can lead to varying degrees of damage to the oral mucosa, the esophagus, and the Gastric mucosa, lining of the stomach. The severity of the injury can be determined by Esophagogastroduodenoscopy, endoscopy of the upper digestive tract, although CT scanning may be more useful to determine whether surgery may be required. During the healing process, Esophageal stricture, strictures of the oesophagus may form, which may require Esophageal dilatation, therapeutic dilatation and insertion of a Esophageal stent, stent. Signs and symptoms Immediate manifestations of caustic substance ingestions include erosions of mucosal surfaces of the gastrointestinal tract or airway (which can cause bleeding if the erosions extend to a blood vessel), mouth and tongue swelling, drooling ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gastroenterology
Gastroenterology (from the Greek gastḗr- “belly”, -énteron “intestine”, and -logía "study of") is the branch of medicine focused on the digestive system and its disorders. The digestive system consists of the gastrointestinal tract, sometimes referred to as the ''GI tract,'' which includes the esophagus, stomach, small intestine and large intestine as well as the accessory organs of digestion which includes the pancreas, gallbladder, and liver. The digestive system functions to move material through the GI tract via peristalsis, break down that material via digestion, absorb nutrients for use throughout the body, and remove waste from the body via defecation. Physicians who specialize in the medical specialty of gastroenterology are called gastroenterologists or sometimes ''GI doctors''. Some of the most common conditions managed by gastroenterologists include gastroesophageal reflux disease, gastrointestinal bleeding, irritable bowel syndrome, irritable bowel dise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
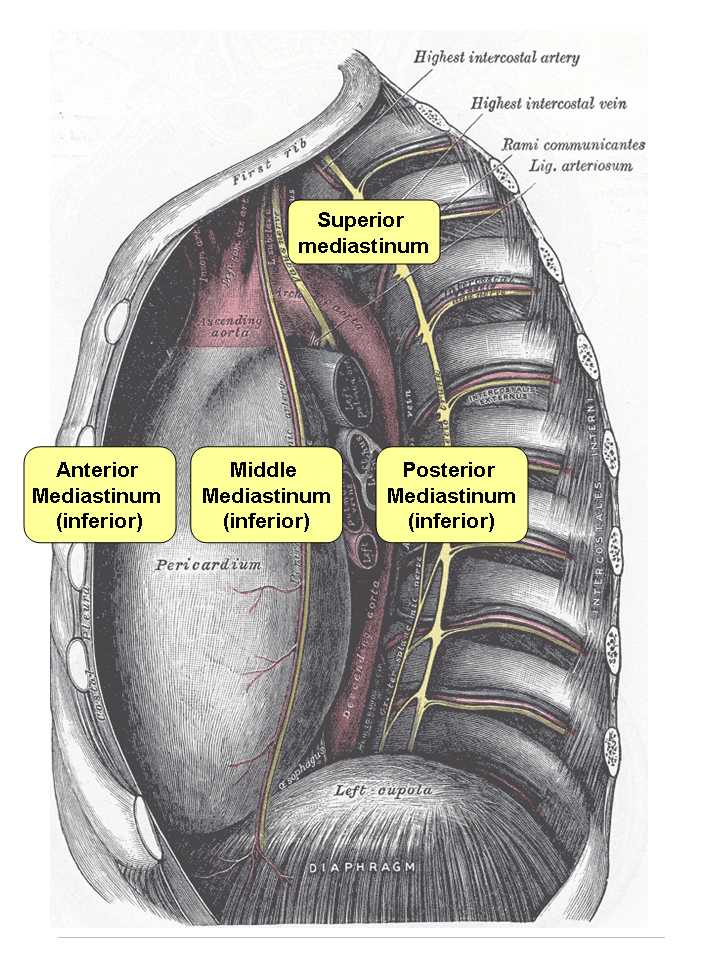
Mediastinitis
Mediastinitis is inflammation of the tissues in the mid-chest, or mediastinum. It can be either Acute (medical), acute or Chronic (medical), chronic. It is thought to be due to four different etiologies: * direct contamination * hematogenous or Lymphatic system, lymphatic spread * extension of infection from the neck or Retroperitoneal space, retroperitoneum * extension from the lung or pleura Acute mediastinitis is usually caused by bacteria and is most often due to perforation of the esophagus. As the infection can progress rapidly, this is considered a serious condition. Chronic Sclerosis (medicine), sclerosing (or fibrosis, fibrosing) mediastinitis, while potentially serious, is caused by a long-standing inflammation of the mediastinum, leading to growth of acellular collagen and fibrous tissue within the chest and around the central vessels and airways. It has a different cause, treatment, and prognosis than acute infectious mediastinitis. Space infections: Pretracheal space ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxalic Acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus ''Oxalis'', commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous. Oxalic acid has much greater acid strength than acetic acid. It is a reducing agent and its conjugate base, known as oxalate (), is a chelating agent for metal cations. Typically, oxalic acid occurs as the dihydrate with the formula . History The preparation of salts of oxalic acid (crab acid) from plants had been known, at least since 1745, when the Dutch botanist and physician Herman Boerhaave isolated a salt from wood sorrel. By 1773, François Pierre Savary of Fribourg, Switzerland had isolated oxalic acid from i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hypochlorite
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an Inorganic chemistry, inorganic chemical compound with the chemical formula, formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may also be viewed as the sodium salt (chemistry), salt of hypochlorous acid. The anhydrous Chemical compound, compound is unstable and may decompose explosively. It can be crystallized as a hydrate, pentahydrate ·5, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated. Sodium hypochlorite is most often encountered as a pale greenish-yellow dilute solution referred to as liquid bleach, which is a household chemical widely used (since the 18th century) as a disinfectant or a bleaching agent. In solution, the compound is unstable and easily decomposes, liberating chlorine, which is the active principle of such products. Sodium hypochlorite is the oldest and still most important chlorine-releasing compounds, chl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash. Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exploit its caustic nature and its reactivity toward acids. An estimated 700,000 to 800,000 tonnes were produced in 2005. KOH is noteworthy as the precursor to most soft and liquid soaps, as well as numerous potassium-containing chemicals. It is a white solid that is dangerously corrosive. Properties and structure KOH exhibits high thermal stability. Because of this high stability and relatively low melting point, it is often melt-cast as pellets or rods, forms that have low surface area and convenient handling properties. These pellets become tacky in air because KOH is hygroscopic. Most commercial samples are ca. 90% pure, the remainder being water and carbonates. Its dissolution in water is strongly exothermic. Concentrated aqueous solut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the manufacture of pulp and paper, textiles, drinking water, soaps and deterge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Hydroxide
Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although the name ammonium hydroxide suggests an alkali with chemical formula, composition , it is actually impossible to isolate samples of NH4OH. The ions and OH− do not account for a significant fraction of the total amount of ammonia except in extremely dilute solutions. Basicity of ammonia in water In aqueous solution, ammonia deprotonation, deprotonates a small fraction of the water to give ammonium and hydroxide according to the following chemical equilibrium, equilibrium: : NH3 + H2O NH4+ + OH−. In a 1 Molar concentration, M ammonia solution, about 0.42% of the ammonia is converted to ammonium, equivalent to pH = 11.63 because [NH4+] = 0.0042 M, [OH−] = 0.0042 M, [NH3] = 0.9958 M, and pH = 14 + log10[OH� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activated Carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that increase the surface area available for adsorption (which is not the same as absorption) or chemical reactions. Activation is analogous to making popcorn from dried corn kernels: popcorn is light, fluffy, and has a surface area that is much larger than the kernels. ''Activated'' is sometimes replaced by ''active''. Due to its high degree of microporosity, one gram of activated carbon has a surface area in excess of as determined by gas adsorption. Charcoal, before activation, has a specific surface area in the range of . An activation level sufficient for useful application may be obtained solely from high surface area. Further chemical treatment often enhances adsorption properties. Activated carbon is usually derived from waste products such as coconut husks; waste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkalis
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline, and less often, alkalescent, is commonly used in English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the Arrhenius definition of a base, and they are still among the most common bases. Etymology The word "alkali" is derived from Arabic ''al qalīy'' (or ''alkali''), meaning ''the calcined ashes'' (see calcination), referring to the original source of alkaline substances. A water-extract of burned plant ashes, called potash and composed mostly of potassium carbonate, was mildly basic. After heating this substance with calcium hydroxide (''slaked lime''), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acids
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequence of database operations that satisfies the ACID properties (which can be perceived as a single logical operation on the data) is called a ''transaction''. For example, a transfer of funds from one bank account to another, even involving multiple changes such as debiting one account and crediting another, is a single transaction. In 1983, Andreas Reuter and Theo Härder coined the acronym ''ACID'', building on earlier work by Jim Gray who named atomicity, consistency, and durability, but not isolation, when characterizing the transaction concept. These four properties are the major guarantees of the transaction paradigm, which has influenced many aspects of development in database systems. According to Gray and Reuter, the IBM Informa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Esophageal Stricture
A benign esophageal stricture, or peptic stricture, is a narrowing or tightening of the esophagus that causes swallowing difficulties. Signs and symptoms Symptoms of esophageal strictures include heartburn, bitter or acid taste in the mouth, choking, coughing, shortness of breath, frequent burping or hiccups, pain or trouble swallowing, throwing up blood, or weight loss. Causes It can be caused by or associated with gastroesophageal reflux disease, esophagitis, a dysfunctional lower esophageal sphincter, disordered motility, lye ingestion, or a hiatal hernia. Strictures can form after esophageal surgery and other treatments such as laser therapy or photodynamic therapy. While the area heals, a scar forms, causing the tissue to pull and tighten, leading to difficulty in swallowing.Ginex, Pamela K., Manjit S. Bains, Jacqueline Hanson, and Bart L. Frazzitta. 100 Questions & Answers About Esophageal Cancer (100 Questions & Answers). New York: Jones and Bartlett, Inc., 2005. Print. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stridor
Stridor (Latin for "creaking or grating noise") is a high-pitched extra-thoracic breath sound resulting from turbulent air flow in the larynx or lower in the bronchial tree. It is different from a stertor which is a noise originating in the pharynx. Stridor is a physical sign which is caused by a narrowed or obstructed airway. It can be inspiratory, expiratory or biphasic, although it is usually heard during inspiration. Inspiratory stridor often occurs in children with croup. It may be indicative of serious airway obstruction from severe conditions such as epiglottitis, a foreign body lodged in the airway, or a laryngeal tumor. Stridor should always command attention to establish its cause. Visualization of the airway by medical experts equipped to control the airway may be needed. Causes Stridor may occur as a result of: * foreign bodies (e.g., aspirated foreign body, aspirated food bolus); * infections (e.g., epiglottitis, retropharyngeal abscess, croup); * subglottic stenos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |