|
Carbosulfan
Carbosulfan is an organic compound adherent to the carbamate class. At normal conditions, it is brown viscous liquid. It is not very stable; it decomposes slowly at room temperature. Its solubility in water is low but it imisciblewith xylene, hexane, chloroform, dichloromethane, methanol and acetone. Carbosulfan is used as an insecticide. The European Union banned use of carbosulfan in 2007. Its oral LD50 for rats is 90 to 250 mg/kg bw, inhalation LC50 is 0.61 mg/L. Carbosulfan is only slightly absorbed through skin (LD50 >2000 mg/kg for rabbits). The mechanism of toxicity is based on reversible inhibition of acetylcholinesterase (as for carbamates generally). Carbosulfan has very low maximum residue limits for use in the EU and UK examples of this can be seen in apples and oranges, where it is 0.05 mg/kg. See also * Carbofuran Carbofuran is a carbamate pesticide, widely used around the world to control insects on a wide variety of field crops, including potatoes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
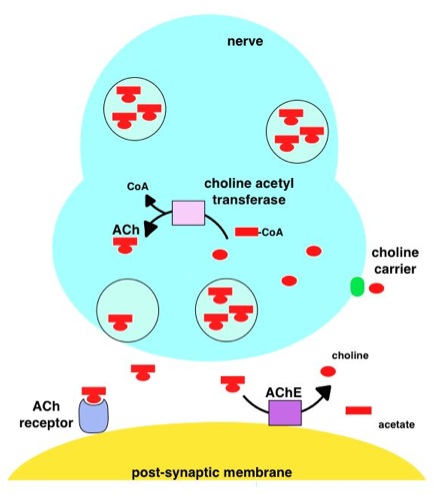
Acetylcholinesterase Inhibitor
Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level and duration of action of acetylcholine in the central nervous system, autonomic ganglia and neuromuscular junctions, which are rich in acetylcholine receptors. Acetylcholinesterase inhibitors are one of two types of cholinesterase inhibitors; the other being butyryl-cholinesterase inhibitors. Acetylcholinesterase is the primary member of the cholinesterase enzyme family. Acetylcholinesterase inhibitors are classified as reversible, irreversible, or quasi-irreversible (also called pseudo-irreversible). Mechanism of action Organophosphates Organophosphates like TEPP and sarin inhibit cholinesterases, enzymes that hydrolyze the neurotransmitter acetylcholine. The active centre of cholinesterases feature two important sites, namely the a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylcholinesterase Inhibitors
Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level and duration of action of acetylcholine in the central nervous system, autonomic ganglia and neuromuscular junctions, which are rich in acetylcholine receptors. Acetylcholinesterase inhibitors are one of two types of cholinesterase inhibitors; the other being butyryl-cholinesterase inhibitors. Acetylcholinesterase is the primary member of the cholinesterase enzyme family. Acetylcholinesterase inhibitors are classified as reversible, irreversible, or quasi-irreversible (also called pseudo-irreversible). Mechanism of action Organophosphates Organophosphates like TEPP and sarin inhibit cholinesterases, enzymes that hydrolyze the neurotransmitter acetylcholine. The active centre of cholinesterases feature two important sites, namely th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LD50
In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a toxic unit that measures the lethal dose of a toxin, radiation, or pathogen. The value of LD50 for a substance is the dose required to kill half the members of a tested population after a specified test duration. LD50 figures are frequently used as a general indicator of a substance's acute toxicity. A lower LD50 is indicative of increased toxicity. The test was created by J.W. Trevan in 1927. The term semilethal dose is occasionally used in the same sense, in particular with translations of foreign language text, but can also refer to a sublethal dose. LD50 is usually determined by tests on animals such as laboratory mice. In 2011, the U.S. Food and Drug Administration approved alternative methods to LD50 for testing the cosmetic drug Botox without animal tests. Conventions The LD50 is usually expressed as the mass of substance administered per unit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catechol Ethers
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. It was first discovered by destructive distillation of the plant extract catechin. About 20,000 tonnes of catechol are now synthetically produced annually as a commodity organic chemical, mainly as a precursor to pesticides, flavors, and fragrances. Catechol occurs as feathery white crystals that are very rapidly soluble in water. Isolation and synthesis Catechol was first isolated in 1839 by Edgar Hugo Emil Reinsch (1809–1884) by distilling it from the solid tannic preparation catechin, which is the residuum of catechu, the boiled or concentrated juice of ''Mimosa catechu'' (''Acacia catechu''). Upon heating catechin above its decomposition point, a substance that Reinsch first named ''Brenz-Katechusäure'' (burned catechu acid) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosulfur Compounds
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, Desulfurization, the removal of which is a Claus process, major focus of oil refineries. Sulfur shares the chalcogen group with oxygen, selenium, and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium, and carbon–tellurium compounds. A classical chemical test for the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
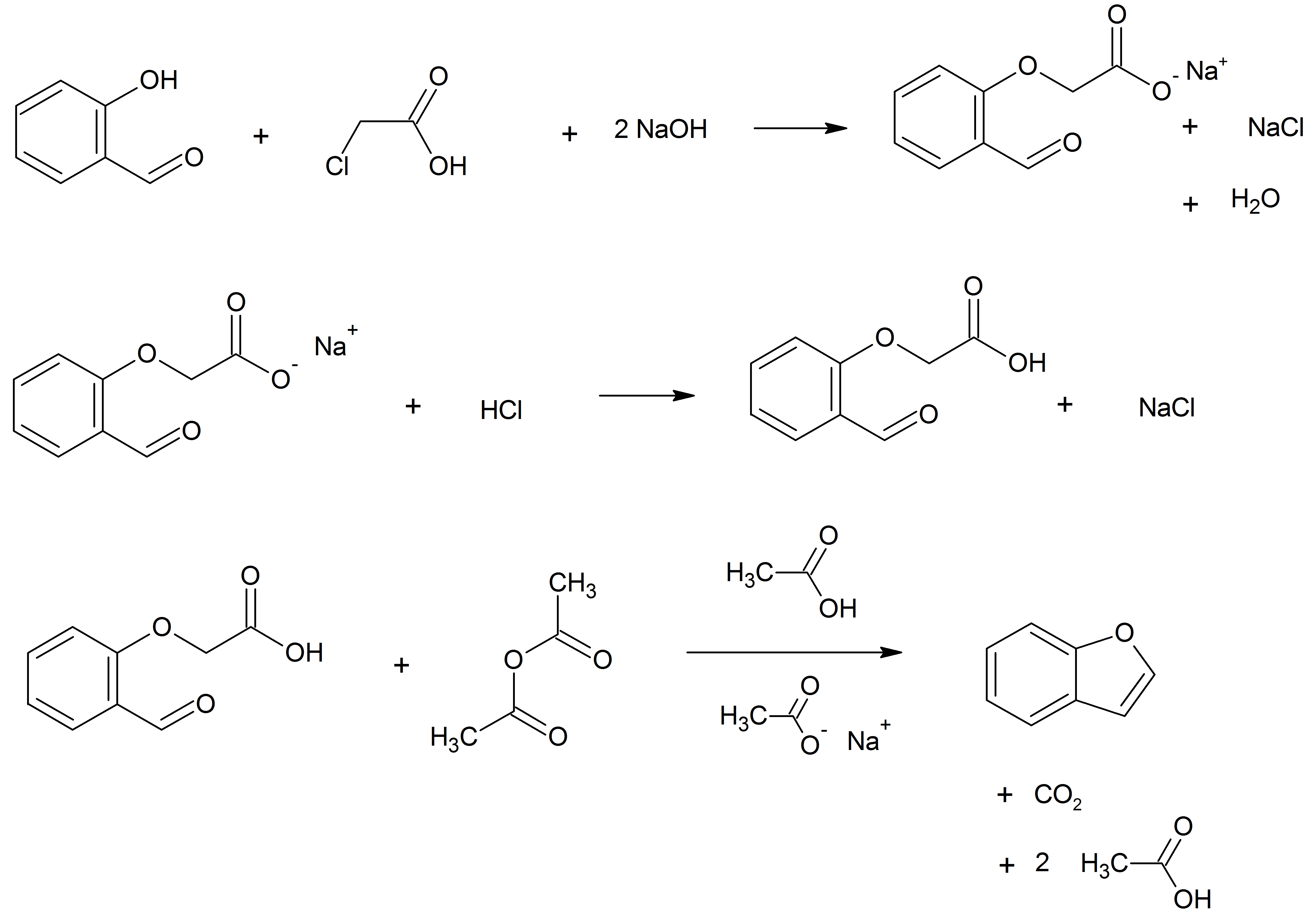
Benzofuran Ethers At The Benzene Ring
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : * Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px * Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amines
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. Aromatic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
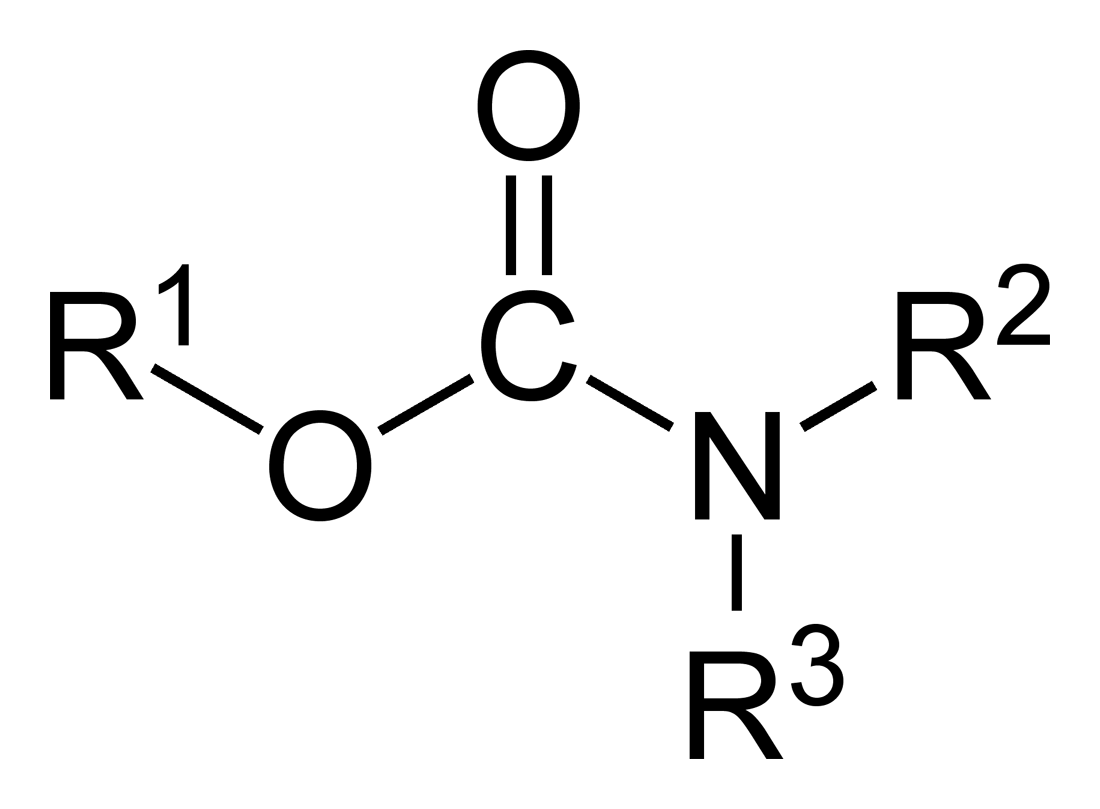
Carbamate Insecticides
In organic chemistry, a carbamate is a category of organic compounds with the general Chemical formula, formula and Chemical structure, structure , which are formally Derivative (chemistry), derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salt (chemistry), salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose units are joined by carbamate groups are an important family of plastics, the polyurethanes. Properties While carbamic acids are unstable, many carbamate esters or salt (chemistry), ionic) are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbofuran
Carbofuran is a carbamate pesticide, widely used around the world to control insects on a wide variety of field crops, including potatoes, corn and soybeans. It is a systemic insecticide, which means that the plant absorbs it through the roots, and from there the plant distributes it throughout its organs where insecticidal concentrations are attained. Carbofuran also has contact activity against pests. It is one of the most toxic pesticides still in use. It is marketed under the trade names Furadan, by FMC Corporation and Curaterr 10 GR, by Bayer among several others. Carbofuran exhibits toxicity mediated by the same mechanism as that of the notorious V-series nerve agents and presents a risk to human health. It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the United States Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylcholinesterase
Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ... that catalysis, catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters: : acetylcholine + H2O = choline + acetate It is found at mainly neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate neurotransmission, synaptic transmission. It belongs to the carboxylesterase family of enzymes. It is the primary target of inhibition by organophosphorus compounds such as nerve agents and pesticides. Enzyme structure and mechani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell ( cytotoxicity) or an organ such as the liver (hepatotoxicity). By extension, the word may be metaphorically used to describe toxic effects on larger and more complex groups, such as the family unit or society at large. Sometimes the word is more or less synonymous with poisoning in everyday usage. A central concept of toxicology is that the effects of a toxicant are dose-dependent; even water can lead to water intoxication when taken in too high a dose, whereas for even a very toxic substance such as snake venom there is a dose below which there is no detectable toxic effect. Toxicity is species-specific, making cross-species analysis problematic. Newer paradigms and metrics are evolving to bypas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |