|
Carbohydrate Acetalisation
In carbohydrate chemistry carbohydrate acetalisation is an organic reaction and a very effective means of providing a protecting group. The example below depicts the acetalisation reaction of D-ribose 1. With acetone or 2,2-dimethoxypropane as the acetalisation reagent the reaction is under thermodynamic reaction control and results in the pentose 2. The latter reagent in itself is an acetal and therefore the reaction is actually a cross-acetalisation. center Kinetic reaction control results from 2-methoxypropene as the reagent. D-ribose in itself is a hemiacetal and in equilibrium with the pyranose Pyranose is a collective term for saccharides that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom. There may be other carbons external to the ring. The name derives from its similarit ... 3. In aqueous solution ribose is 75% pyranose and 25% furanose and a different acetal 4 is formed. Selective acetalization of carb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbohydrate Chemistry
Carbohydrate chemistry is a subdiscipline of chemistry primarily concerned with the detection, synthesis, structure, and function of carbohydrates. Due to the general structure of carbohydrates, their synthesis is often preoccupied with the selective formation of glycosidic linkages and the selective reaction of hydroxyl groups; as a result, it relies heavily on the use of protecting groups. Monosaccharides Individual saccharide residues are termed monosaccharides. Carbohydrate synthesis Carbohydrate synthesis is a sub-field of organic chemistry concerned specifically with the generation of natural and unnatural carbohydrate structures. This can include the synthesis of monosaccharide residues or structures containing more than one monosaccharide, known as oligosaccharides. Glycosidic bond formation * Chemical glycosylation * Fischer glycosidation * Glycosyl halide * Koenigs-Knorr reaction Protecting groups * Carbohydrate acetalisation * Trimethylsilyl * Benzyl Ether * para ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organic Photochemistry, photochemical reactions and organic redox reaction, redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protecting Group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis. In many preparations of delicate organic compounds, some specific parts of their molecules cannot survive the required reagents or chemical environments. Then, these parts, or groups, must be protected. For example, lithium aluminium hydride is a highly reactive but useful reagent capable of reducing esters to alcohols. It will always react with carbonyl groups, and this cannot be discouraged by any means. When a reduction of an ester is required in the presence of a carbonyl, the attack of the hydride on the carbonyl has to be prevented. For example, the carbonyl is converted into an acetal, which does not react with hydrides. The acetal is then called a protecting group for the carbonyl. After the step involving the hydride is complete, the acet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetalisation
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other (a "symmetric acetal") or not (a "mixed acetal"). Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry. The term ketal is sometimes used to identify structures associated with ketones (both R groups organic fragments rather than hydrogen) rather than aldehydes and, historically, the term acetal was used specifically for the aldehyde-related cases (having at least one hydrogen in place of an R on t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribose
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally-occurring form, , is a component of the ribonucleotides from which RNA is built, and so this compound is necessary for coding, decoding, regulation and expression of genes. It has a structural analog, deoxyribose, which is a similarly essential component of DNA. is an unnatural sugar that was first prepared by Emil Fischer and Oscar Piloty in 1891. It was not until 1909 that Phoebus Levene and Walter Jacobs recognised that was a natural product, the enantiomer of Fischer and Piloty's product, and an essential component of nucleic acids. Fischer chose the name "ribose" as it is a partial rearrangement of the name of another sugar, arabinose, of which ribose is an epimer at the 2' carbon; both names also relate to gum arabic, from which arabinose was first isolated and from which they prepared . Like most sugars, ribose exists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour. Acetone is miscible with water and serves as an important organic solvent in its own right, in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate (and from that PMMA) as well as bisphenol A.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in |
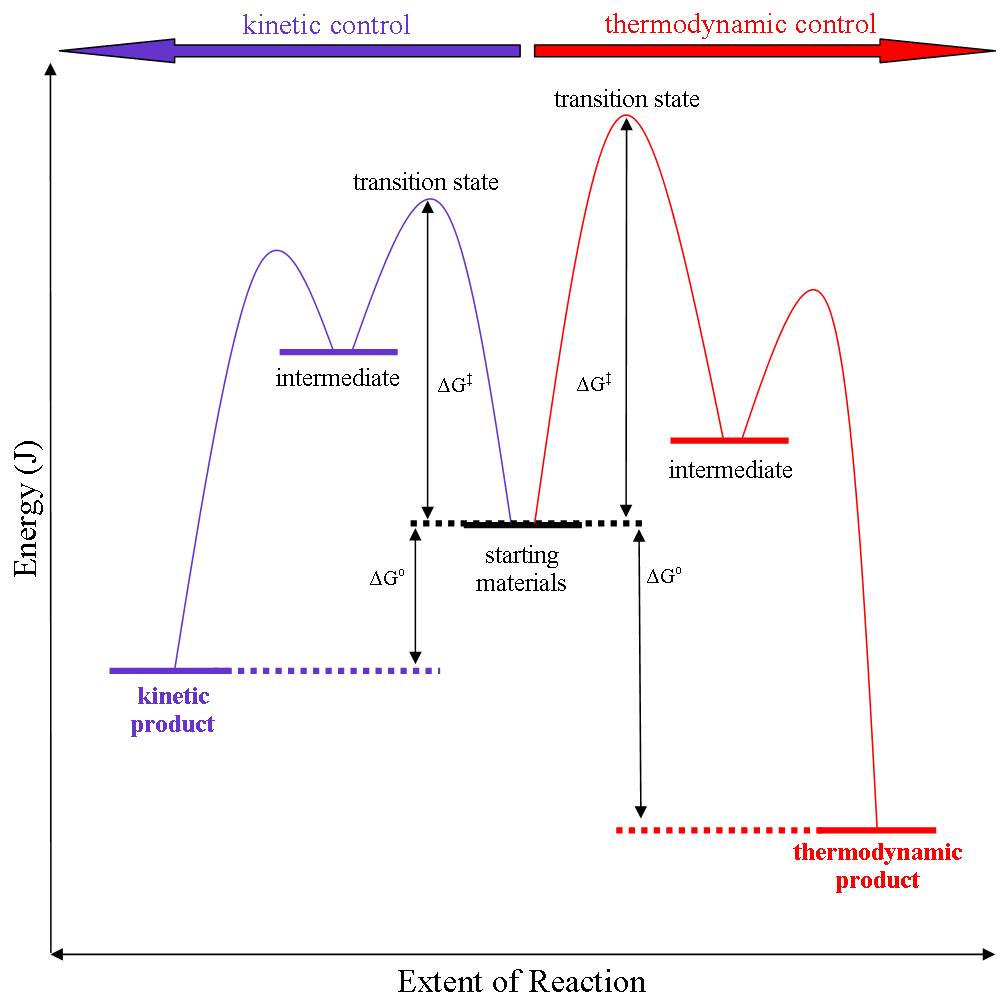
Thermodynamic Reaction Control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity or stereoselectivity. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favoured under kinetic control and B is the thermodynamic product and is favoured under thermodynamic control.Introduction to Organic Chemistry I, Seth Robert Elsheimer, Blackwell Publishing, 2000 The conditions of the reaction, such as temperature, pressure, or solvent, affect which reaction pathway may be favored: either the kinetically controlled or the thermodynamically controlled one. Note this is only true if the activation energy of the two pathways differ, with one pathway having a lower ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentose
In chemistry, a pentose is a monosaccharide (simple sugar) with five carbon atoms. The chemical formula of many pentoses is , and their molecular weight is 150.13 g/mol.-Ribose . PubChem compound webpage, accessed on 2010-02-06. Pentoses are very important in . is a constituent of , and the related molecule, , is a constituent of [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |