|
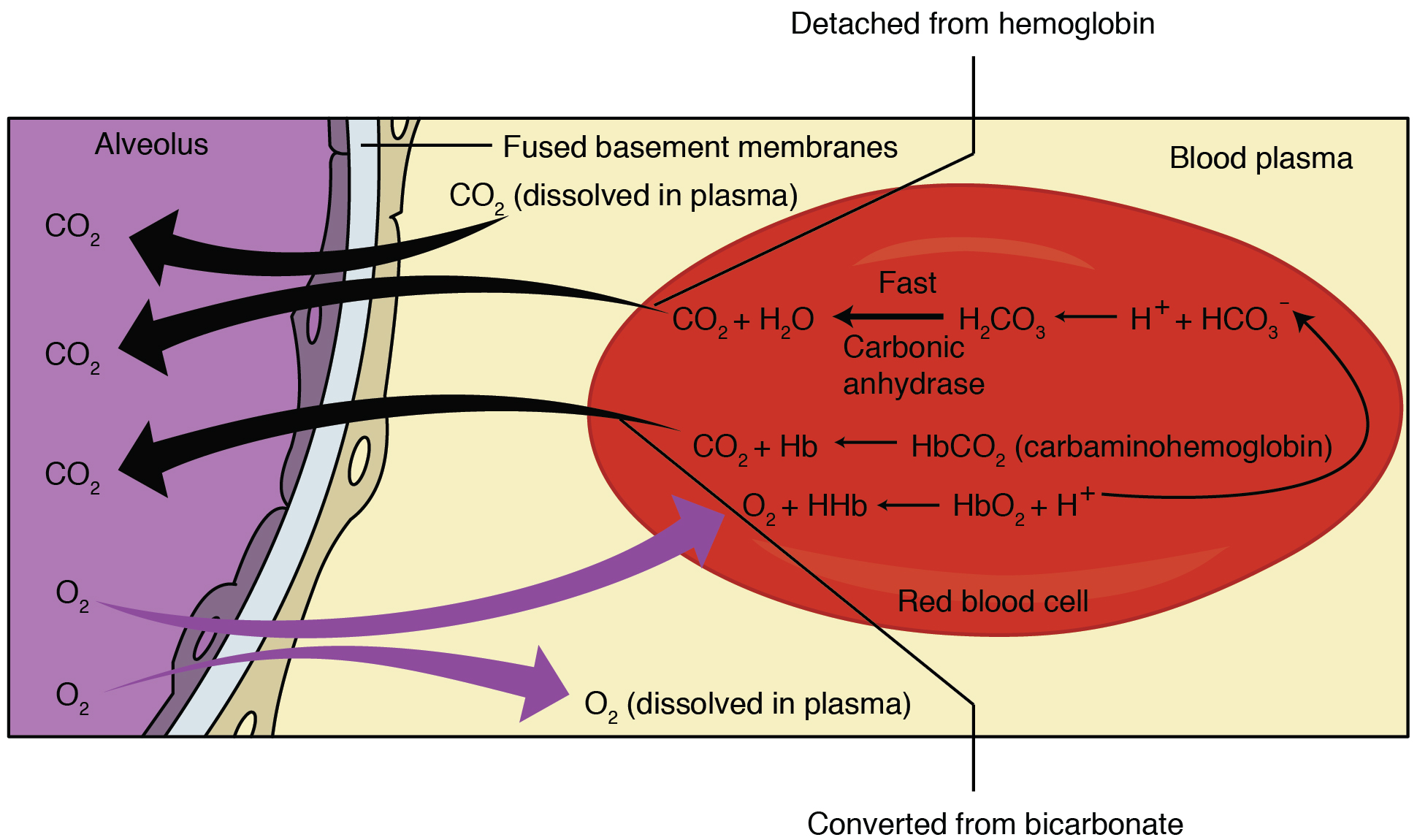
Carbaminohemoglobin
Carbaminohemoglobin (carbaminohaemoglobin BrE) (CO2Hb, also known as carbhemoglobin and carbohemoglobin) is a compound of hemoglobin and carbon dioxide, and is one of the forms in which carbon dioxide exists in the blood. Twenty-three percent of carbon dioxide is carried in blood this way (70% is converted into bicarbonate by carbonic anhydrase and then carried in plasma, 7% carried as free CO2, dissolved in plasma). Synthesis When the tissues release carbon dioxide into the bloodstream, around 10% is dissolved into the plasma. The rest of the carbon dioxide is carried either directly or indirectly by hemoglobin. Approximately 10% of the carbon dioxide carried by hemoglobin is in the form of carbaminohemoglobin. This carbaminohemoglobin is formed by the reaction between carbon dioxide and an amino (-NH2) residue from the globin molecule, resulting in the formation of a carbamino residue (-NH.COO−). The rest of the carbon dioxide is transported in the plasma as bicarbonate ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haldane Effect
The Haldane effect is a property of hemoglobin first described by John Scott Haldane, within which oxygenation of blood in the lungs displaces carbon dioxide from hemoglobin, increasing the removal of carbon dioxide. Consequently, oxygenated blood has a reduced affinity for carbon dioxide. Thus, the Haldane effect describes the ability of hemoglobin to carry increased amounts of carbon dioxide (CO2) in the deoxygenated state as opposed to the oxygenated state. A high concentration of CO2 facilitates dissociation of oxyhemoglobin. Carbaminohemoglobin Carbon dioxide travels through the blood in three different ways. One of these ways is by binding to amino groups, creating carbamino compounds. Amino groups are available for binding at the N-terminals and at side-chains of arginine and lysine residues in hemoglobin. When carbon dioxide binds to these residues carbaminohemoglobin is formed. This amount of carbaminohemoglobin formed is inversely proportional to the amount of oxygen at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin
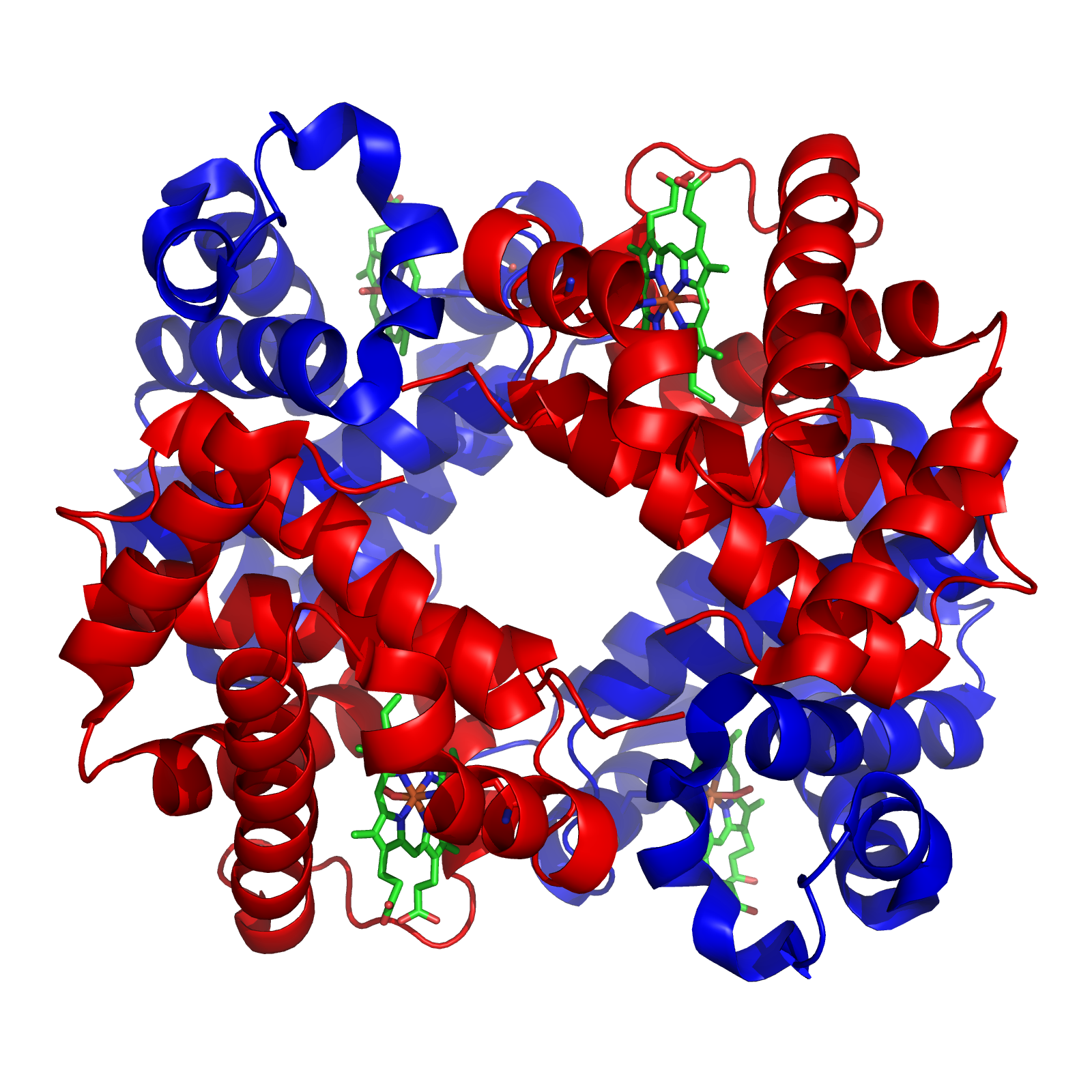
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs (''e.g.'' lungs or gills) to the rest of the body (''i.e.'' tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12to 20grams of hemoglobin in every 100mL of blood. In mammals, the chromoprotein makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL O2 per gram, which increases the total blood oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs (''e.g.'' lungs or gills) to the rest of the body (''i.e.'' tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12to 20grams of hemoglobin in every 100mL of blood. In mammals, the chromoprotein makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL O2 per gram, which increases the total blood oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the circulatory system is also known as ''peripheral blood'', and the blood cells it carries, ''peripheral blood cells''. Blood is composed of blood cells suspended in blood plasma. Plasma, which constitutes 55% of blood fluid, is mostly water (92% by volume), and contains proteins, glucose, mineral ions, hormones, carbon dioxide (plasma being the main medium for excretory product transportation), and blood cells themselves. Albumin is the main protein in plasma, and it functions to regulate the colloidal osmotic pressure of blood. The blood cells are mainly red blood cells (also called RBCs or erythrocytes), white blood cells (also called WBCs or leukocytes) and platelets (also called thrombocytes). The most abundant cells in vertebrate blo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula . Bicarbonate serves a crucial biochemical role in the physiological pH buffering system. The term "bicarbonate" was coined in 1814 by the English chemist William Hyde Wollaston. The name lives on as a trivial name. Chemical properties The bicarbonate ion (hydrogencarbonate ion) is an anion with the empirical formula and a molecular mass of 61.01 daltons; it consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a hydrogen atom attached to one of the oxygens. It is isoelectronic with nitric acid . The bicarbonate ion carries a negative one formal charge and is an amphiprotic species which has both acidic and basic properties. It is both the conjugate base of carbonic acid ; and the conjugate acid of , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonic Anhydrase
The carbonic anhydrases (or carbonate dehydratases) () form a family of enzymes that catalyze the interconversion between carbon dioxide and water and the dissociated ions of carbonic acid (i.e. bicarbonate and hydrogen ions). The active site of most carbonic anhydrases contains a zinc ion. They are therefore classified as metalloenzymes. The enzyme maintains acid-base balance and helps transport carbon dioxide. Carbonic anhydrase helps maintain acid–base homeostasis, regulate pH, and fluid balance. Depending on its location, the role of the enzyme changes slightly. For example, carbonic anhydrase produces acid in the stomach lining. In the kidney, the control of bicarbonate ions influences the water content of the cell. The control of bicarbonate ions also influences the water content in the eyes. Inhibitors of carbonic anhydrase are used to treat glaucoma, the excessive build-up of water in the eyes. Blocking this enzyme shifts the fluid balance in the eyes to reduce flui ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bohr Effect
The Bohr effect is a phenomenon first described in 1904 by the Danish physiologist Christian Bohr. Hemoglobin's oxygen binding affinity (see oxygen–haemoglobin dissociation curve) is inversely related both to acidity and to the concentration of carbon dioxide. That is, the Bohr effect refers to the shift in the oxygen dissociation curve caused by changes in the concentration of carbon dioxide or the pH of the environment. Since carbon dioxide reacts with water to form carbonic acid, an increase in CO2 results in a decrease in blood pH, resulting in hemoglobin proteins releasing their load of oxygen. Conversely, a decrease in carbon dioxide provokes an increase in pH, which results in hemoglobin picking up more oxygen. Experimental discovery In the early 1900s, Christian Bohr was a professor at the University of Copenhagen in Denmark, already well known for his work in the field of respiratory physiology. He had spent the last two decades studying the solubility of oxygen, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydron (chemistry)
In chemistry, the hydron, informally called proton, is the cationic form of atomic hydrogen, represented with the symbol . The general term "hydron", endorsed by the IUPAC, encompasses cations of hydrogen regardless of their isotopic composition: thus it refers collectively to protons (1H+) for the protium isotope, deuterons (2H+ or D+) for the deuterium isotope, and tritons (3H+ or T+) for the tritium isotope. Unlike most other ions, the hydron consists only of a bare atomic nucleus. The negatively charged counterpart of the hydron is the hydride anion, . Properties Solute properties Other things being equal, compounds that readily donate hydrons (Brønsted acids, see below) are generally polar, hydrophilic solutes and are often soluble in solvents with high relative static permittivity (dielectric constants). Examples include organic acids like acetic acid (CH3COOH) or methanesulfonic acid (CH3SO3H). However, large nonpolar portions of the molecule may attenuate these pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
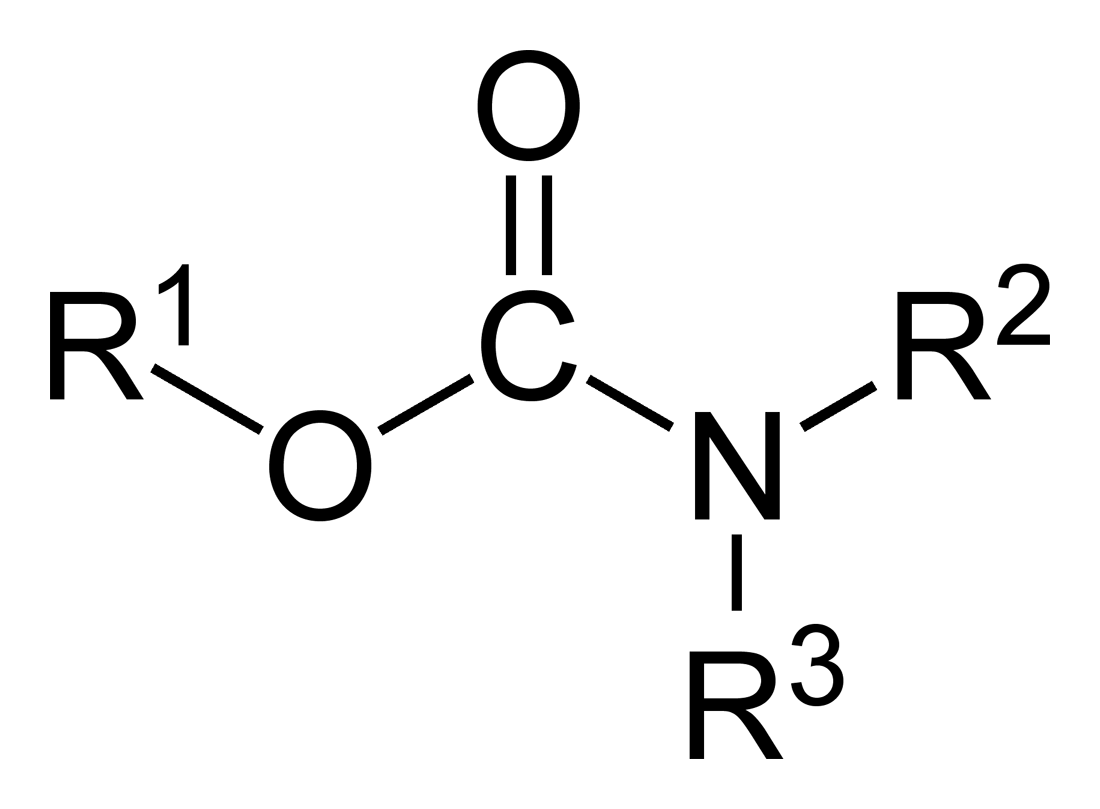
Carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose units are joined by carbamate groups are an important family of plastics, the polyurethanes. Properties While carbamic acids are unstable, many carbamate esters or ionic) are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to an ammonium carbamate/carbonate solution will precipitate some calcium carbonate immediatel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |