|
Capmatinib
Capmatinib, sold under the brand name Tabrecta, is a medication for the treatment of adults with metastatic non-small cell lung cancer (NSCLC) whose tumors have a mutation that leads to the exon 14 skipping of the ''MET'' gene, which codes for the membrane receptor HGFR, as detected by an FDA-approved test. The most common adverse reactions are peripheral edema, nausea, fatigue, vomiting, dyspnea, and decreased appetite. Non-small cell lung cancer (NSCLC) is a disease in which malignant cancer cells form in the tissues of the lung. It is the most common type of lung cancer with up to 90% of all lung carcinomas falling into the non-small cell category. NSCLC occurs when healthy cells become abnormal and grow rapidly. One danger of this form of cancer is that there's a high likelihood that the cancer cells will spread from the lungs to other organs and body parts. Cancer metastasis consists of a sequential series of events, and MET exon 14 skipping is recognized as a critical even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Priority Review
Priority review is a program of the United States Food and Drug Administration (FDA) to expedite the review process for drugs that are expected to have a particularly great impact on the treatment of a disease. The priority review voucher program is a program that grants a voucher for priority review to a drug developer as an incentive to develop treatments for disease indications with limited profitability. Priority review vouchers are currently earned by pharmaceutical companies for the development and approval of drugs treating neglected tropical diseases, rare pediatric diseases, and "medical countermeasures" for terrorism. The voucher can be used for future drugs that could have wider indications for use, but the company is required to pay a fee (approximately $2.8 million) to use the voucher. When seeking approval for a drug, manufacturers can apply to the FDA for priority review. This is granted when a drug is intended to treat a serious condition and would "provide a si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breakthrough Therapy
Breakthrough therapy is a United States Food and Drug Administration designation that expedites drug development that was created by Congress under Section 902 of the 9 July 2012 Food and Drug Administration Safety and Innovation Act. The FDA's "breakthrough therapy" designation is not intended to imply that a drug is actually a "breakthrough" or that there is high-quality evidence of treatment efficacy for a particular condition; rather, it allows the FDA to grant priority review to drug candidates if preliminary clinical trials indicate that the therapy may offer substantial treatment advantages over existing options for patients with serious or life-threatening diseases. The FDA has other mechanisms for expediting the review and approval process for promising drugs, including fast track designation, accelerated approval, and priority review. Requirements A breakthrough therapy designation can be assigned to a drug if "it is a drug which is intended alone or in combination with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antineoplastic Drugs
Chemotherapy (often abbreviated to chemo and sometimes CTX or CTx) is a type of cancer treatment that uses one or more anti-cancer drugs (chemotherapeutic agents or alkylating agents) as part of a standardized chemotherapy regimen. Chemotherapy may be given with a curative intent (which almost always involves combinations of drugs) or it may aim to prolong life or to reduce symptoms ( palliative chemotherapy). Chemotherapy is one of the major categories of the medical discipline specifically devoted to pharmacotherapy for cancer, which is called ''medical oncology''. The term ''chemotherapy'' has come to connote non-specific usage of intracellular poisons to inhibit mitosis (cell division) or induce DNA damage, which is why inhibition of DNA repair can augment chemotherapy. The connotation of the word chemotherapy excludes more selective agents that block extracellular signals ( signal transduction). The development of therapies with specific molecular or genetic targets, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee For Medicinal Products For Human Use
The Committee for Medicinal Products for Human Use (CHMP), formerly known as Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding medicinal products for human use. See also * Committee for Medicinal Products for Veterinary Use The Committee for Medicinal Products for Veterinary Use (CVMP) is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding veterinary medicines. Text was copied from this source which is © ... References External links Committee for Medicinal Products for Human Use (CHMP) Health and the European Union {{eu-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breakthrough Therapy
Breakthrough therapy is a United States Food and Drug Administration designation that expedites drug development that was created by Congress under Section 902 of the 9 July 2012 Food and Drug Administration Safety and Innovation Act. The FDA's "breakthrough therapy" designation is not intended to imply that a drug is actually a "breakthrough" or that there is high-quality evidence of treatment efficacy for a particular condition; rather, it allows the FDA to grant priority review to drug candidates if preliminary clinical trials indicate that the therapy may offer substantial treatment advantages over existing options for patients with serious or life-threatening diseases. The FDA has other mechanisms for expediting the review and approval process for promising drugs, including fast track designation, accelerated approval, and priority review. Requirements A breakthrough therapy designation can be assigned to a drug if "it is a drug which is intended alone or in combination with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy in many countries and has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug research and development. In the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to encourage development of drugs which would otherwise lack sufficient profit motive to attract corporate research budgets and personnel. Definition According to the US Food and Drug Administration (FDA), an orphan drug is defined as one "intended for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Accelerated Approval (FDA)
The United States Food and Drug Administration (FDA) initiated the FDA Accelerated Approval Program in 1992 to allow faster approval of drugs for serious conditions that fill an unmet medical need. The faster approval relies on use of surrogate endpoints. Drug approval typically requires clinical trials with endpoints that demonstrate a clinical benefit, such as increased survival for cancer patients. Drugs with accelerated approval can initially be tested in clinical trials that use a surrogate endpoint, or something that is thought to predict clinical benefit. Surrogate endpoints typically require less time, and in the case of a cancer patient, it is much faster to measure a reduction in tumor size, for example, than overall patient survival. Drugs approved under the FDA Accelerated Approval Program still need to be tested in clinical trials using endpoints that demonstrate clinical benefit, and those trials are known as phase 4 confirmatory trials. If the drug later proves unabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-small Cell Lung Cancer
Non-small-cell lung cancer (NSCLC) is any type of epithelial lung cancer other than small-cell lung carcinoma (SCLC). NSCLC accounts for about 85% of all lung cancers. As a class, NSCLCs are relatively insensitive to chemotherapy, compared to small-cell carcinoma. When possible, they are primarily treated by surgical resection with curative intent, although chemotherapy has been used increasingly both preoperatively ( neoadjuvant chemotherapy) and postoperatively (adjuvant chemotherapy). Types The most common types of NSCLC are squamous-cell carcinoma, large-cell carcinoma, and adenocarcinoma, but several other types occur less frequently. A few of the less common types are pleomorphic, carcinoid tumor, salivary gland carcinoma, and unclassified carcinoma. All types can occur in unusual histologic variants and as mixed cell-type combinations. Nonsquamous-cell carcinoma almost occupies the half of NSCLC. In the tissue classification, the central type contains about one-ninth. So ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organogenesis
Organogenesis is the phase of embryonic development that starts at the end of gastrulation and continues until birth. During organogenesis, the three germ layers formed from gastrulation (the ectoderm, endoderm, and mesoderm) form the internal organs of the organism. The cells of each of the three germ layers undergo differentiation, a process where less-specialized cells become more-specialized through the expression of a specific set of genes. Cell differentiation is driven by cell signaling cascades. Differentiation is influenced by extracellular signals such as growth factors that are exchanged to adjacent cells which is called juxtracrine signaling or to neighboring cells over short distances which is called paracrine signaling. Intracellular signals consist of a cell signaling itself (autocrine signaling), also play a role in organ formation. These signaling pathways allow for cell rearrangement and ensure that organs form at specific sites within the organism. The organoge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
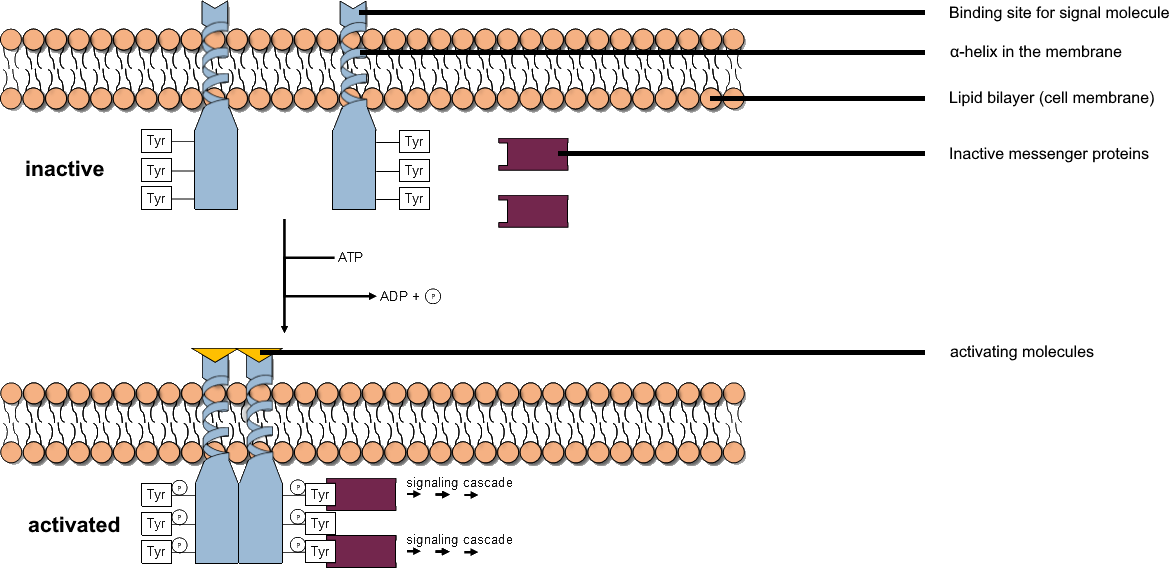
Tyrosine Kinase
A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an "on" or "off" switch in many cellular functions. Tyrosine kinases belong to a larger class of enzymes known as protein kinases which also attach phosphates to other amino acids such as serine and threonine. Phosphorylation of proteins by kinases is an important mechanism for communicating signals within a cell (signal transduction) and regulating cellular activity, such as cell division. Protein kinases can become mutated, stuck in the "on" position, and cause unregulated growth of the cell, which is a necessary step for the development of cancer. Therefore, kinase inhibitors, such as imatinib and osimertinib, are often effective cancer treatments. Most tyrosine kinases have an associated protein tyrosine phosphatase, which removes the phosphate group. Reaction Protein kinases are a group of enzymes that possess a catal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |