|
Triazines
Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is . They exist in three isomeric forms, 1,3,5-triazines being common. Structure The triazines have planar six-membered benzene-like ring but with three carbons replaced by nitrogens. The three isomers of triazine are distinguished by the positions of their nitrogen atoms, and are referred to as 1,2,3-triazine, 1,2,4-triazine, and 1,3,5-triazine. Other aromatic nitrogen heterocycles are pyridines with one ring nitrogen atom, diazines with 2 nitrogen atoms in the ring, triazoles with 3 nitrogens in a 5-membered ring, and tetrazines with 4 ring nitrogen atoms. Uses Melamine A well known triazine is melamine (2,4,6-triamino-1,3,5-triazine). With three amino substituents, melamine is a precursor to commercial resins. Guanamines are closely related to melamine, except with one amino substituent replaced by an organic group. This difference is exploited in the use of guanamine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanamine
In organic chemistry, a guanamine is an organic compound with the formula (H2NC)2N3CR. They are heterocycles of the triazine class. Guanamines are closely related to melamine ((H2NC)3N3), except with one amino substituent replaced by an organic group. With two amines, guanamines are bifunctional, whereas melamine is trifunctional. This difference is exploited in the use of guanamines to modify the crosslink density in melamine resins. They are white or colorless solids of low toxicity. Some popular guanamines are the phenyl, methyl and nonyl derivatives, called benzoguanamine, acetoguanamine, and capriguanamine. They are all prepared by the condensation of cyanoguanidine with the corresponding nitrile In organic chemistry, a nitrile is any organic compound that has a functional group. The name of the compound is composed of a base, which includes the carbon of the , suffixed with "nitrile", so for example is called " propionitrile" (or pr ...: :(H2N)2C=NCN + RCN ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atrazine
Atrazine ( ) is a Organochlorine compound, chlorinated herbicide of the triazine class. It is used to prevent pre-emergence broadleaf weeds in crops such as maize (corn), soybean and sugarcane and on turf, such as golf courses and residential lawns. Atrazine's primary manufacturer is Syngenta and it is one of the most widely used herbicides in the United States, Canadian, and Australian agriculture. Its use was banned in the European Union in 2004, when the EU found groundwater levels exceeding the limits set by regulators, and Syngenta could not show that this could be prevented nor that these levels were safe.European Commission.2004/248/EC: Commission Decision of 10 March 2004 concerning the non-inclusion of atrazine in Annex I to Council Directive 91/414/EEC and the withdrawal of authorisations for plant protection products containing this active substance (Text with EEA relevance) (notified under document number C(2004) 731) Decision 2004/248/EC - Official Journal L 078, Decis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanuric Chloride
Cyanuric chloride is an organic compound with the formula (NCCl)3. This white solid is the chlorinated derivative of 1,3,5-triazine. It is the trimer of cyanogen chloride. Cyanuric chloride is the main precursor to the popular but controversial herbicide atrazine. Production Cyanuric chloride is prepared in two steps from hydrogen cyanide via the intermediacy of cyanogen chloride, which is trimerized at elevated temperatures over a carbon catalyst: :HCN + Cl2 → ClCN + HCl : In 2005, approximately 200,000 tons were produced.Klaus Huthmacher, Dieter Most "Cyanuric Acid and Cyanuric Chloride" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. . Industrial uses It is estimated that 70% of cyanuric chloride is used in the preparation of the triazine-class pesticides, especially atrazine. Such reactions rely on the easy displacement of the chloride with nucleophiles such as amines: :(ClCN)3 + 2 RNH2 → (RNHCN)(ClCN)2 + RNH3+Cl− Other tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetoguanamine
Acetoguanamine is an organic compound with the chemical formula (CNH2)2CCH3N3. It is related to melamine but with one amino group replaced by methyl. Acetoguanamine is used in the manufacturing of melamine resins. Unlike melamine ((CNH2)3N3), acetoguanamine is not a crosslinker. The "aceto" prefix is historical, the compound does not contain an acetyl group. A related compound is benzoguanamine. The compound is prepared by condensation of cyanoguanidine with acetonitrile: :(H2N)2C=NCN + MeCN → (CNH2)2(CMe)N3 Safety LD50 In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a toxic unit that measures the lethal dose of a given substance. The value of LD50 for a substance is the dose requ ... (oral, rats) is 2740 mg/kg. References Triazines {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melamine
Melamine is an organic compound with the formula C3H6N6. This white solid is a trimer (chemistry), trimer of cyanamide, with a 1,3,5-Triazine, 1,3,5-triazine skeleton. Like cyanamide, it contains 66% nitrogen by mass, and its derivatives have fire retardant, fire-retardant properties due to its release of nitrogen gas when burned or charred. Melamine can be combined with formaldehyde and other agents to produce melamine resins. Such resins are characteristically durable thermosetting plastic used in high–pressure decorative laminates such as Formica (plastic), Formica, melamine dinnerware including cooking utensils, plates, and plastic products, laminate flooring, and dry erase boards. Melamine foam is used as insulation and soundproofing material, and in polymeric cleaning products such as Magic Eraser. Melamine-formaldehyde resin tableware was evaluated by the Taiwan Consumers' Foundation to have 20,000 parts per billion of free melamine that could migrate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoguanamine
Benzoguanamine is an organic Chemical compound, compound with the chemical formula (CNH2)2(CC6H5)N3. It is related to melamine but with one amino group replaced by phenyl. Benzoguanamine is used in the manufacturing of melamine resins. Unlike melamine ((CNH2)3N3), benzoguanamine is not a crosslinker. The "benzo" prefix is historical, as the compound contains phenyl, not a benzo group. A related compound is acetoguanamine. The compound is prepared by the condensation reaction of cyanoguanidine with benzonitrile. Safety LD50 (oral, rats) is 1470 mg/kg. References Triazines {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom. Ionic cyanides contain the cyanide anion . This anion is extremely poisonous. Soluble cyanide salts such as sodium cyanide (NaCN), potassium cyanide (KCN) and tetraethylammonium cyanide () are highly toxic. Covalent cyanides contain the group, and are usually called nitriles if the group is linked by a single covalent bond to carbon atom. For example, in acetonitrile , the cyanide group is bonded to methyl . In tetracyanomethane , four cyano groups are bonded to carbon. Although nitriles generally do not release cyanide ions, the cyanohydrins do and are thus toxic. The cyano group may be covalently bonded to atoms different than carbon, e.g., in cyanogen azide , phosphorus tricyanide and trimethylsilyl cyanide . Hydrogen cyanide, or , is a highly volatile toxic liquid tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The name of the compound is composed of a base, which includes the carbon of the , suffixed with "nitrile", so for example is called " propionitrile" (or propanenitrile). The prefix '' cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons. Inorganic compounds containing the group are not called nitriles, but cyanides instead. Though both nitriles and cyanides can be derived from cyanide salts, most nitriles are not nearly as toxic. Structure and basic properties The N−C−C geom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist Carl Wilhelm Scheele is credited with having discovered the chemical composition of purified hydrogen sulfide in 1777. Hydrogen sulfide is toxic to humans and most other animals by inhibiting cellular respiration in a manner similar to hydrogen cyanide. When it is inhaled or its salts are ingested in high amounts, damage to organs occurs rapidly with symptoms ranging from breathing difficulties to convulsions and death. Despite this, the human body produces small amounts of this sulfide and its mineral salts, and uses it as a signalling molecule. Hydrogen sulfide is often produced from the microbial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers; this process is commonly known as anaerobic digestio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Herbicide
Herbicides (, ), also commonly known as weed killers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page for EPA reports on pesticide use ihere Selective herbicides control specific weed species while leaving the desired crop relatively unharmed, while non-selective herbicides (sometimes called "total weed killers") kill plants indiscriminately. The combined effects of herbicides, nitrogen fertilizer, and improved cultivars has increased yields (per acre) of major crops by three to six times from 1900 to 2000. In the United States in 2012, about 91% of all herbicide usage, was determined by weight applied, in agriculture. In 2012, world pesticide expenditures totaled nearly US$24.7 billion; herbicides were about 44% of those sales and constituted the biggest portion, followed by insecticides, fungicides, and fumigants. Herbicide is also used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
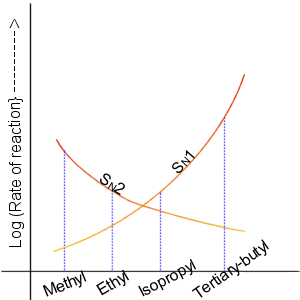
Nucleophilic Substitution
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The molecule that contains the electrophile and the leaving functional group is called the substrate. The most general form of the reaction may be given as the following: :\text\mathbf + \ce + \text\mathbf The electron pair (:) from the nucleophile (Nuc) attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br under basic conditions, where the attacking nucleophile is hydroxyl () and the leaving group is bromide (). :O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |