|
Isotopes Of Germanium
Germanium (32Ge) has five naturally occurring isotopes, 70Ge, 72Ge, 73Ge, 74Ge, and 76Ge. Of these, 76Ge is very slightly radioactive, decaying by double beta decay with a half-life of 1.78 × 1021 years (130 billion times the age of the universe). Stable 74Ge is the most common isotope, having a natural abundance of approximately 36%. 76Ge is the least common with a natural abundance of approximately 7%. When bombarded with alpha particles, the isotopes 72Ge and 76Ge will generate stable 75As and 77Se, releasing high energy electrons in the process.Via a set of two reactions:4He + 72Ge → 75Se + 1n, 75Se decays by electron capture to 75As with a half-life of 120 days76Ge + 1n → 77Ge, which then undergoes beta decay to 77As with a half-life of 11.3 hours, which in turn undergoes beta decay to 77Se with a half-life of 39 hours At least 27 radioisotopes have also been synthesized ranging in atomic mass from 58 to 89. The most stable of these is 68Ge, decaying by electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors silicon and tin. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature. Because it seldom appears in high concentration, germanium was discovered comparatively late in the discovery of the elements. Germanium ranks near fiftieth in relative abundance of the elements in the Earth's crust. In 1869, Dmitri Mendeleev predicted its existence and some of its properties from its position on his periodic table, and called the element ekasilicon. In 1886, Clemens Winkler at Freiberg University found the new element, along with silver and sulfur, in the mineral argyrodite. Winkler named the element after his country, Germany. Germanium is mined primarily from sphalerite (the primary ore of zinc), though germanium is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
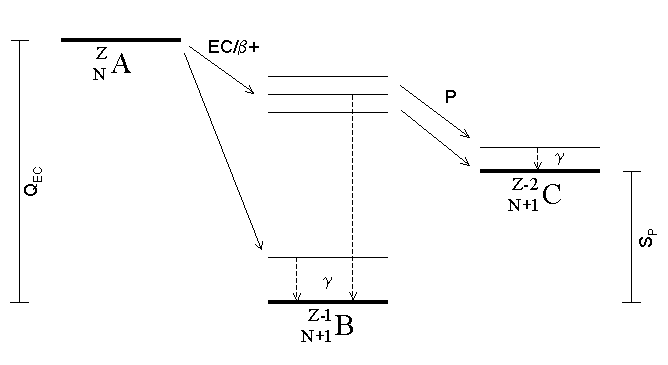
Beta Decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in so-called ''positron emission''. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron em ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chinese Physics C
''Chinese Physics C'' (CPC) is a monthly peer-reviewed scientific journal published by the Chinese Physical Society along with the Institute of High Energy Physics, Chinese Academy of Sciences. CPC is hosted online by IOP Publishing. It reports on research into the theory, experiment and applications of particle physics, nuclear physics and astrophysics. The journal was established in 1977 as ''High Energy Physics and Nuclear Physics'', and renamed to its present title in 2008. Its current impact factor is 2.145 (2020). ''Chinese Physics C'' is part of the SCOAP3 initiative. In 2014 and 2016, the journal hosted the publication of the Particle Data Group's bi-annual ''Review of Particle Physics'', the most highly cited article in the field of particle physics Particle physics or high energy physics is the study of fundamental particles and forces that constitute matter and radiation. The fundamental particles in the universe are classified in the Standard Model as fermions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Particle Data Group
The Particle Data Group (or PDG) is an international collaboration of particle physicists that compiles and reanalyzes published results related to the properties of particles and fundamental interactions. It also publishes reviews of theoretical results that are phenomenologically relevant, including those in related fields such as cosmology. The PDG currently publishes the ''Review of Particle Physics'' and its pocket version, the ''Particle Physics Booklet'', which are printed biennially as books, and updated annually via the World Wide Web. In previous years, the PDG has published the ''Pocket Diary for Physicists'', a calendar with the dates of key international conferences and contact information of major high energy physics institutions, which is now discontinued. PDG also further maintains the standard numbering scheme for particles in event generators, in association with the event generator authors. ''Review of Particle Physics'' The ''Review of Particle Physics'' (former ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (''t''1/2) for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primordial Nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the interstellar medium from which the solar system was formed, and were formed in, or after, the Big Bang, by nucleosynthesis in stars and supernovae followed by mass ejection, by cosmic ray spallation, and potentially from other processes. They are the stable nuclides plus the long-lived fraction of radionuclides surviving in the primordial solar nebula through planet accretion until the present; 286 such nuclides are known. Stability All of the known 251 stable nuclides, plus another 35 nuclides that have half-lives long enough to have survived from the formation of the Earth, occur as primordial nuclides. These 35 primordial radionuclides represent isotopes of 28 separate elements. Cadmium, tellurium, xenon, neodymium, samarium, osmium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomeric Transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have half-lives 100 to 1000 times longer than the half-lives of the excited nuclear states that decay with a "prompt" half life (ordinarily on the order of 10−12 seconds). The term "metastable" is usually restricted to isomers with half-lives of 10−9 seconds or longer. Some references recommend 5 × 10−9 seconds to distinguish the metastable half life from the normal "prompt" gamma-emission half-life. Occasionally the half-lives are far longer than this and can last minutes, hours, or years. For example, the nuclear isomer survives so long (at least 1015 years) that it has never been observed to decay spontaneously. The half-life of a nuclear isomer can even exceed that of the ground state of the same nuclide, as shown by as well as , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino. : : or when written as a nuclear reaction equation, ^_e + ^_p -> ^_n + ^_ ν_e Since this single emitted neutrino carries the entire decay energy, it has this single characteristic energy. Similarly, the momentum of the neutrino emission causes the daughter atom to recoil with a single characteristic momentum. The resulting daughter nuclide, if it is in an excited state, then transitions to its ground state. Usually, a gamma ray is emitted during this transition, but nuclear de-excitation may also take place by internal conversion. Following capture of an inner electron from the atom, an outer electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Beta Decay
In nuclear physics, double beta decay is a type of radioactive decay in which two neutrons are simultaneously transformed into two protons, or vice versa, inside an atomic nucleus. As in single beta decay, this process allows the atom to move closer to the optimal ratio of protons and neutrons. As a result of this transformation, the nucleus emits two detectable beta particles, which are electrons or positrons. The literature distinguishes between two types of double beta decay: ''ordinary'' double beta decay and ''neutrinoless'' double beta decay. In ordinary double beta decay, which has been observed in several isotopes, two electrons and two electron antineutrinos are emitted from the decaying nucleus. In neutrinoless double beta decay, a hypothesized process that has never been observed, only electrons would be emitted. History The idea of double beta decay was first proposed by M. Goeppert-Mayer in 1935. In 1937, E. Majorana demonstrated that all results of beta decay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Emission
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a nucleus. It occurs in the most neutron-rich/proton-deficient nuclides, and also from excited states of other nuclides as in photoneutron emission and beta-delayed neutron emission. As only a neutron is lost by this process the number of protons remains unchanged, and an atom does not become an atom of a different element, but a different isotope of the same element. Neutrons are also produced in the spontaneous and induced fission of certain heavy nuclides. Spontaneous neutron emission As a consequence of the Pauli exclusion principle, nuclei with an excess of protons or neutrons have a higher average energy per nucleon. Nuclei with a sufficient excess of neutrons have a greater energy than the combination of a free neutron and a nucleus with one less neutron, and therefore can decay by neutron emission. Nuclei which can decay by this process are described as lying beyond the neutron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton Emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state (or a low-lying isomer) of very proton-rich nuclei, in which case the process is very similar to alpha decay. For a proton to escape a nucleus, the proton separation energy must be negative—the proton is therefore unbound, and tunnels out of the nucleus in a finite time. Proton emission is not seen in naturally occurring isotopes; proton emitters can be produced via nuclear reactions, usually using linear particle accelerators. Although prompt (i.e. not beta-delayed) proton emission was observed from an isomer in cobalt-53 as early as 1969, no other proton-emitting states were found until 1981, when the proton radioactive ground states of lutetium-15 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties. The term isotope is formed from the Greek roots isos ( ἴσος "equal") and topos ( τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in 1913 in a suggestion to the British chemist Frederick Soddy. The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |