|
Drug Discovery
In the fields of medicine, biotechnology, and pharmacology, drug discovery is the process by which new candidate medications are discovered. Historically, drugs were discovered by identifying the active ingredient from traditional remedies or by serendipitous discovery, as with penicillin. More recently, chemical libraries of synthetic small molecules, natural products, or extracts were screened in intact cells or whole organisms to identify substances that had a desirable therapeutic effect in a process known as classical pharmacology. After sequencing of the human genome allowed rapid cloning and synthesis of large quantities of purified proteins, it has become common practice to use high throughput screening of large compounds libraries against isolated biological targets which are hypothesized to be disease-modifying in a process known as reverse pharmacology. Hits from these screens are then tested in cells and then in animals for efficacy. Modern drug discovery i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biotechnology
Biotechnology is a multidisciplinary field that involves the integration of natural sciences and Engineering Science, engineering sciences in order to achieve the application of organisms and parts thereof for products and services. Specialists in the field are known as biotechnologists. The term ''biotechnology'' was first used by Károly Ereky in 1919 to refer to the production of products from raw materials with the aid of living organisms. The core principle of biotechnology involves harnessing biological systems and organisms, such as bacteria, yeast, and plants, to perform specific tasks or produce valuable substances. Biotechnology had a significant impact on many areas of society, from medicine to agriculture to environmental science. One of the key techniques used in biotechnology is genetic engineering, which allows scientists to modify the genetic makeup of organisms to achieve desired outcomes. This can involve inserting genes from one organism into another, and con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affinity (pharmacology)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from Latin ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
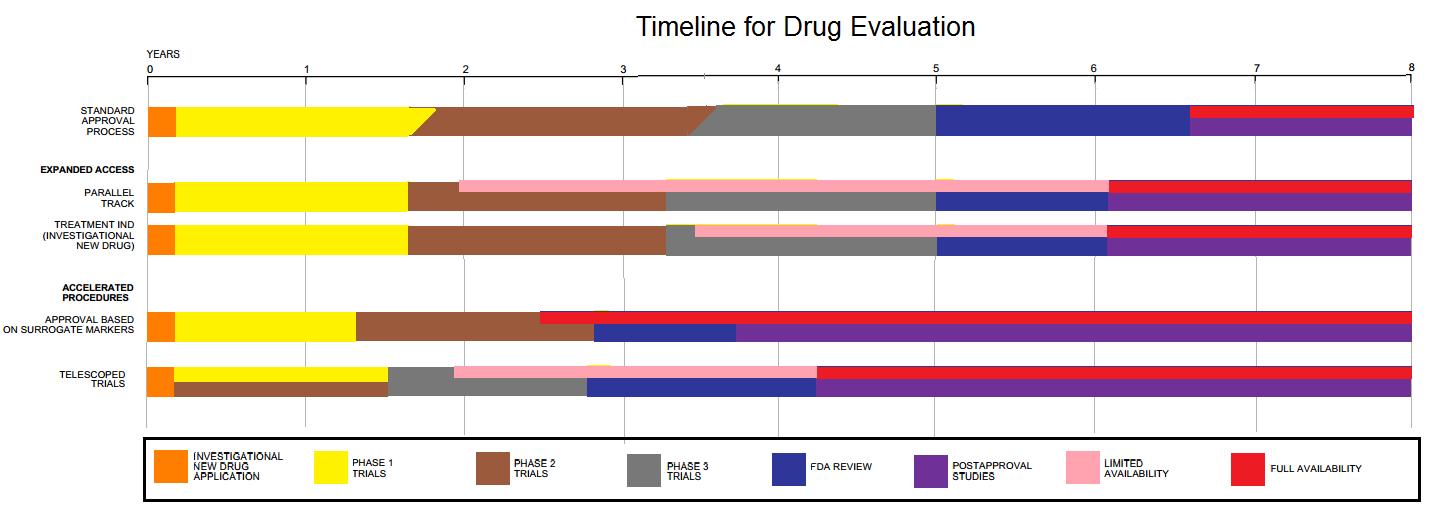
New Drug Application
The Food and Drug Administration's (FDA) New Drug Application (NDA) is the vehicle in the United States through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing. Some 30% or less of initial drug candidates proceed through the entire multi-year process of drug development, concluding with an approved NDA, if successful. The goals of the NDA are to provide enough information to permit FDA reviewers to establish the complete history of the candidate drug. Among facts needed for the application are: * Patent and manufacturing information * Drug safety and specific effectiveness for its proposed use(s) when used as directed * Reports on the design, compliance, and conclusions of completed clinical trials by the Institutional Review Board * Drug susceptibility to substance abuse, abuse * Proposed labeling (package insert) and directions for use Exceptions to this process include voter driven initiatives for Medical cannabis, medic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
New Molecular Entity
A new chemical entity (NCE) is, according to the U.S. Food and Drug Administration, a novel, small, chemical molecule drug that is undergoing clinical trials or has received a first approval (not a new use) by the FDA in any other application submitted under section 505(b) of the Federal Food, Drug, and Cosmetic Act. A new molecular entity (NME) is a broader term that encompasses both an NCE or an NBE (New Biological Entity). Definition An active moiety is a molecule or ion, excluding those appended portions of the molecule that cause the drug to be an ester, salt (including a salt with hydrogen or coordination bonds), or other noncovalent derivative (such as a complex, chelate, or clathrate) of the molecule, responsible for the physiological or pharmacological action of the drug substance. An NCE is a molecule developed by the innovator company in the early drug discovery stage, which after undergoing clinical trials could translate into a drug that could be a treatment fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Research And Development
Research and development (R&D or R+D), known in some countries as OKB, experiment and design, is the set of innovative activities undertaken by corporations or governments in developing new services or products. R&D constitutes the first stage of development of a potential new service or the production process. Although R&D activities may differ across businesses, the primary goal of an R&D department is to new product development, develop new products and services. R&D differs from the vast majority of corporate activities in that it is not intended to yield immediate profit, and generally carries greater risk and an uncertain return on investment. R&D is crucial for acquiring larger shares of the market through new products. ''R&D&I'' represents R&D with innovation. Background New product design and development is often a crucial factor in the survival of a company. In a global industrial landscape that is changing fast, firms must continually revise their design and range of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Loan Guarantee
A loan guarantee, in finance, is a promise by one party (the guarantor) to assume the debt obligation of a borrower if that borrower defaults. A guarantee can be limited or unlimited, making the guarantor liable for only a portion or all of the debt. Private loan guarantees There are two main types: # Guarantor mortgages # Unsecured guarantor loan Guarantor mortgages Popular with young borrowers who do not have a large deposit saved and need to borrow up to 100% of the property value to purchase a property. Generally, their parents will provide a guarantee to the lender to cover any shortfall in the event of default. There are three main types # Guarantor Mortgage: – generally, a parent or close family member will guarantee the mortgage debt and will cover the repayment obligations should the borrower default. # Family offset mortgage: typically, a parent or grandparent will put their savings into an account linked to the borrower’s mortgage. They do not get any intere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmaceutical Industry
The pharmaceutical industry is a medical industry that discovers, develops, produces, and markets pharmaceutical goods such as medications and medical devices. Medications are then administered to (or self-administered by) patients for curing or preventing disease or for alleviating symptoms of illness or injury. Pharmaceutical companies may deal in generic drugs, branded drugs, or both, in different contexts. Generic materials are without the involvement of intellectual property, whereas branded materials are protected by chemical patents. The industry's various subdivisions include distinct areas, such as manufacturing biologics and total synthesis. The industry is subject to a variety of laws and regulations that govern the patenting, efficacy testing, safety evaluation, and marketing of these drugs. The global pharmaceutical market produced treatments worth a total of $1,228.45 billion in 2020. The sector showed a compound annual growth rate (CAGR) of 1.8% in 2021, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capital Intensity
Capital intensity is the amount of fixed or real capital present in relation to other factors of production, especially labor. At the level of either a production process or the aggregate economy, it may be estimated by the capital to labor ratio, such as from the points along a capital/labor isoquant. The inverse of capital intensity is labor intensity. Capital intensity is sometimes associated with industrialism, while labor intensity is sometimes associated with agrarianism. Growth The use of tools and machinery makes labor more effective, so rising capital intensity (or " capital deepening") pushes up the productivity of labor. Capital intensive societies tend to have a higher standard of living over the long run. Calculations made by Robert Solow claimed that economic growth was mainly driven by technological progress (productivity growth) rather than inputs of capital and labor. However recent economic research has invalidated that theory, since Solow did not properly consi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human subject research, human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, pharmaceutical drug, drugs, medical nutrition therapy, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received institutional review board, health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small Pilot experiment, pi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process—from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials—to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. These h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation. By definition, when a medication is administered intravenously, its bioavailability is 100%. However, when a medication is administered via routes other than intravenous, its bioavailability is lower due to intestinal epithelium absorption and first-pass metabolism. Thereby, mathematically, bioavailability equals the ratio of comparing the area under the plasma drug concentration curve versus time (AUC) for the extravascular formulation to the AUC for the intravascular formulation. AUC is used because AUC is proportional to the dose that has entered the systemic circulation. Bioavailability of a drug is an average value; to take population variability into account, deviation range is shown as ±. To ensure that the drug taker who has poor absorption is dosed appropriately, the bottom value of the deviation range is employed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biological Half-life
Biological half-life (elimination half-life, pharmacological half-life) is the time taken for concentration of a drug, biological substance (such as a medication) to decrease from its maximum concentration (chemistry), concentration (Cmax (pharmacology), Cmax) to half of Cmax in the blood plasma. It is denoted by the abbreviation t_. This is used to measure the removal of things such as metabolites, drugs, and signalling molecules from the body. Typically, the biological half-life refers to the body's natural detoxification (cleansing) through liver metabolism and through the excretion of the measured substance through the kidneys and intestines. This concept is used when the rate of removal is roughly Exponential function, exponential. In a medical context, half-life explicitly describes the time it takes for the blood plasma concentration of a substance to halve (''plasma half-life'') its steady-state when circulating in the full blood of an organism. This measurement is usefu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |