|
Counter-current Exchange
Countercurrent exchange is a mechanism occurring in nature and mimicked in industry and engineering, in which there is a crossover of some property, usually heat or some chemical, between two flowing bodies flowing in opposite directions to each other. The flowing bodies can be liquids, gases, or even solid powders, or any combination of those. For example, in a distillation column, the vapors bubble up through the downward flowing liquid while exchanging both heat and mass. The maximum amount of heat or mass transfer that can be obtained is higher with countercurrent than co-current (parallel) exchange because countercurrent maintains a slowly declining difference or gradient (usually temperature or concentration difference). In cocurrent exchange the initial gradient is higher but falls off quickly, leading to wasted potential. For example, in the adjacent diagram, the fluid being heated (exiting top) has a higher exiting temperature than the cooled fluid (exiting bottom) that w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is also often used to refer to the thermal energy contained in a system as a component of its internal energy and that is reflected in the temperature of the system. For both uses of the term, heat is a form of energy. An example of formal vs. informal usage may be obtained from the right-hand photo, in which the metal bar is "conducting heat" from its hot end to its cold end, but if the metal bar is considered a thermodynamic system, then the energy flowing within the metal bar is called internal energy, not heat. The hot metal bar is also transferring heat to its surroundings, a correct statement for both the strict and loose meanings of ''heat''. Another example of informal usage is the term '' heat content'', used despite the fact that p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Circulus Arteriosus Schaf
Circulus may refer to: * ''Circulus'' (album), 1970, by Chick Corea *Circulus (band), a British psychedelic folk and progressive rock band * ''Circulus'' (gastropod), a genus of sea snails *Circulus (theory), a socioeconomics doctrine *Circulus (zoology) A circulus is a rarely occurring reptilian social group where there is interaction and personal exchange between individuals. Members will often protect and defend young, even if not of direct genetic linkage. ''Circulus'' is a Latin based term; ..., a type of reptilian social group See also * {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Heat Capacity
In thermodynamics, the specific heat capacity (symbol ) of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity. Informally, it is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. The SI unit of specific heat capacity is joule per kelvin per kilogram, J⋅kg−1⋅K−1. For example, the heat required to raise the temperature of of water by is , so the specific heat capacity of water is . Specific heat capacity often varies with temperature, and is different for each state of matter. Liquid water has one of the highest specific heat capacities among common substances, about at 20 °C; but that of ice, just below 0 °C, is only . The specific heat capacities of iron, granite, and hydrogen gas are about 449 J⋅kg−1⋅K−1, 790 J⋅kg−1⋅K−1, and 14300 J⋅kg−1⋅K−1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solution (chemistry)
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. These surrounded solute particles then move away from the solid solute and out into the solution. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical syn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmotic Pressure
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane. It is also defined as the measure of the tendency of a solution to take in a pure solvent by osmosis. Potential osmotic pressure is the maximum osmotic pressure that could develop in a solution if it were separated from its pure solvent by a semipermeable membrane. Osmosis occurs when two solutions containing different concentrations of solute are separated by a selectively permeable membrane. Solvent molecules pass preferentially through the membrane from the low-concentration solution to the solution with higher solute concentration. The transfer of solvent molecules will continue until equilibrium is attained. Theory and measurement Jacobus van 't Hoff found a quantitative relationship between osmotic pressure and solute concentration, expressed in the following equation: :\Pi = icRT where \Pi is osmotic p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
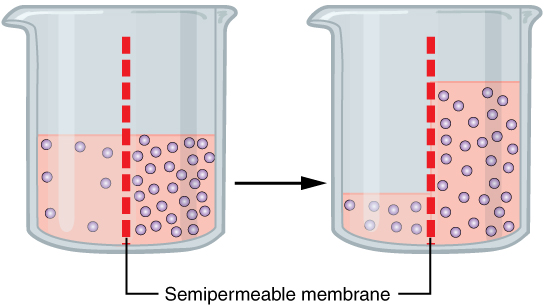
Osmosis
Osmosis (, ) is the spontaneous net movement or diffusion of solvent molecules through a selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of low water potential (region of higher solute concentration), in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane (permeable to the solvent, but not the solute) separating two solutions of different concentrations. Osmosis can be made to do work. Osmotic pressure is defined as the external pressure required to be applied so that there is no net movement of solvent across the membrane. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity. Osmosis is a vital process in biological systems, as biological membranes are semiperm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semi Permeable Membrane
Semipermeable membrane is a type of biological or synthetic, polymeric membrane that will allow certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of the egg. Biological membranes are selectively permeable, with the passage of molecules controlled by facilitated diffusion, passive transport or active transport regulated by proteins embedded in the membrane. Biological membranes An example of a biological semi-permeable membrane is th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Delta T 1
Delta commonly refers to: * Delta (letter) (Δ or δ), a letter of the Greek alphabet * River delta, at a river mouth * D (NATO phonetic alphabet: "Delta") * Delta Air Lines, US * Delta variant of SARS-CoV-2 that causes COVID-19 Delta may also refer to: Places Canada * Delta, British Columbia ** Delta (electoral district), a federal electoral district ** Delta (provincial electoral district) * Delta, Ontario United States * Mississippi Delta * Delta, Alabama * Delta Junction, Alaska * Delta, Colorado * Delta, Illinois * Delta, Iowa * Delta, Kentucky * Delta, Louisiana * Delta, Missouri * Delta, North Carolina * Delta, Ohio * Delta, Pennsylvania * Sacramento–San Joaquin River Delta, California * Delta, Utah * Delta, Wisconsin, a town * Delta (community), Wisconsin * Delta County (other) Elsewhere * Delta Island, Antarctica * Delta Stream, Antarctica * Delta, Minas Gerais, Brazil * Nile Delta, Egypt * Delta, Thessaloniki, Greece * Delta State, Nigeria * Delta, A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Comparison Of Con- And Counter-current Flow Exchange
Comparison or comparing is the act of evaluating two or more things by determining the relevant, comparable characteristics of each thing, and then determining which characteristics of each are similar to the other, which are different, and to what degree. Where characteristics are different, the differences may then be evaluated to determine which thing is best suited for a particular purpose. The description of similarities and differences found between the two things is also called a comparison. Comparison can take many distinct forms, varying by field: To compare things, they must have characteristics that are similar enough in relevant ways to merit comparison. If two things are too different to compare in a useful way, an attempt to compare them is colloquially referred to in English as "comparing apples and oranges." Comparison is widely used in society, in science and in the arts. General usage Comparison is a natural activity, which even animals engage in when deci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |