|
Conjugate Addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarity, unless the alkene is activated with special substituents. With α,β-unsaturated carbonyl compounds such as cyclohexenone it can be deduced from resonance structures that the β position is an electrophilic site which can react with a nucleophile. The negative charge in these structures is stored as an alkoxide anion. Such a nucleophilic addition is called a nucleophilic conjugate addition or 1,4-nucleophilic addition. The most important active alkenes are the aforementioned conjugated carbonyls and acrylonitriles. Reaction mechanism Conjugate addition is the vinylogous counterpart of direct nucleophilic addition. A nucleophile reacts with a α,β-unsaturated carbonyl compound in the β position. The negative charge carried by the n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid, is deprotonation.) Some examples include *The protonation of water by sulfuric acid: *:H2SO4 + H2O H3O+ + *The protonation of isobutene in the formation of a carbocation: *:(CH3)2C=CH2 + HBF4 (CH3)3C+ + *The protonation of ammonia in the formation of ammonium chloride from ammonia and hydrogen chloride: *:NH3( g) + HCl( g) → NH4Cl( s) Protonation is a fundamental chemical reaction and is a step in many stoichiometric and catalytic processes. Some ions and molecules can undergo more than one protonation and are labeled polybasic, which is true of many biological macromolecules. Protonation and deprotonation (removal of a proton) occur in most acid–base reactions; they are the core of most acid–base reaction theories. A Brønst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates. : The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and the root ''amine''. This can be compared with enol, which is a functional group containing both alkene (''en''-) and alcohol (-''ol''). Enamines are considered to be nitrogen analogs of enols. If one of the nitrogen substituents is a hydrogen atom, H, it is the tautomeric form of an imine. This usually will rearrange to the imine; however there are several exceptions (such as aniline). The enamine-imine tautomerism may be considered analogous to the keto-enol tautomerism. In both cases, a hydrogen atom switches its location between the heteroatom (oxygen or nitrogen) and the second carbon atom. Enamines are both good nucleophiles and good bases. Their behavior as carbon-based nucleophiles is explained with reference to the following reson ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stork Enamine Reaction
The Stork enamine alkylation involves the addition of an enamine to a Michael acceptor (e.g, an α,β -unsaturated carbonyl compound) or another electrophilic alkylation reagent to give an alkylated iminium product, which is hydrolyzed by dilute aqueous acid to give the alkylated ketone or aldehyde. Since enamines are generally produced from ketones or aldehydes, this overall process (known as the Stork enamine synthesis) constitutes a selective monoalkylation of a ketone or aldehyde, a process that may be difficult to achieve directly. The Stork enamine synthesis: # formation of an enamine from a ketone # addition of the enamine to an alpha, beta-unsaturated aldehyde or ketone # hydrolysis of the enamine back to a ketone The reaction also applies to acyl halides as electrophiles, which results in the formation of 1,3-diketones (Stork acylation). It is also effective for activated sp3 alkyl electrophiles, including benzylic, allylic/propargylic, α-carbonyl (e.g., bromoacet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds. Bonding and structure Enolate anions are electronically related to allyl anions. The anionic charge is delocalized over the oxygen and the two carbon sites. Thus they have the character of both an alkoxide and a carbanion. Although they are often drawn as being simple salts, in fact they adopt complicated structures often featuring aggregates. Preparation Deprotonation of enolizable ketones, aromatic alcohols, aldehydes, and esters gives enolates. With strong bases, the deprotonation is quantitative. Typically enolates are generated from using lithium diisopropylamide (LDA). Often, as in conventional Claisen condensations, Mannich reactions, and aldol condensations, enolates are generated in low concentrations with alkoxide bases. Under such conditions, they exist in low concent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michael Reaction
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds. The Michael addition is an important atom-economical method for diastereoselective and enantioselective C–C bond formation, and many asymmetric variants exist : In this general Michael addition scheme, either or both of R and R' on the nucleophile (the Michael donor) represent electron-withdrawing substituents such as acyl, cyano, nitro, or sulfone groups, which make the adjacent methylene hydrogen acidic enough to form a carbanion when reacted with the base, ''B:''. For the alkene (the Michael acceptor), the R" substituent is usually a carbonyl, which makes the compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
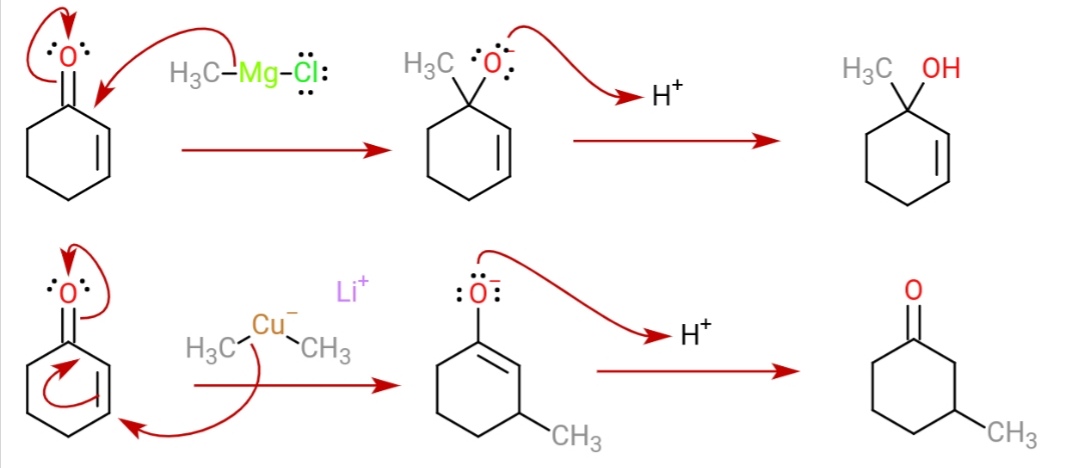
Gilman Reagent
A Gilman reagent is a lithium and copper ( diorganocopper) reagent compound, R2CuLi, where R is an alkyl or aryl. These reagents are useful because, unlike related Grignard reagents and organolithium reagents, they react with organic halides to replace the halide group with an R group (the Corey–House reaction). Such displacement reactions allow for the synthesis of complex products from simple building blocks. Reactions These reagents were discovered by Henry Gilman and coworkers. Lithium dimethylcopper (CH3)2CuLi can be prepared by adding copper(I) iodide to methyllithium in tetrahydrofuran at −78 °C. In the reaction depicted below, the Gilman reagent is a methylating reagent reacting with an alkyne in a conjugate addition, and the negative charge is trapped in a nucleophilic acyl substitution with the ester group forming a cyclic enone. Due to the softness of the nucleophile, they do 1,4 addition on conjugated enones, rather than 1,2 addition. : Struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethylaluminum Cyanide
Diethylaluminum cyanide ("Nagata's reagent") is the organoaluminum compound with formula ((C2H5)2AlCN)n. This colorless compound is usually handled as a solution in toluene. It is a reagent for the hydrocyanation of α,β-unsaturated ketones. Synthesis Diethylaluminum cyanide was originally generated by treatment of triethylaluminum with a slight excess of hydrogen cyanide. The product is typically stored in ampoules because it is highly toxic. It dissolves in toluene, benzene, hexane and isopropyl ether. It undergoes hydrolysis readily and is not compatible with protic solvents. :Et3Al + HCN → 1/n (Et2AlCN)n + EtH Structure Diethylaluminum cyanide has not been examined by X-ray crystallography, although other diorganoaluminum cyanides have been. Diorganylaluminum cyanides have the general formula (R2AlCN)''n'', and they exist as cyclic trimers (''n'' = 3) or tetramers (''n'' = 4). In these oligomers, one finds AlCN---Al linkages. One compound similar to diethyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocyanation Of Unsaturated Carbonyls
In organic chemistry, hydrocyanation is a process for conversion of alkenes to nitriles. The reaction involves the addition of hydrogen cyanide and requires a catalyst. This conversion is conducted on an industrial scale for the production of precursors to nylon. Hydrocyanation of unactivated alkenes Industrially, hydrocyanation is commonly performed on alkenes catalyzed by nickel complexes of phosphite () ligands. A general reaction is shown:Piet W.N.M. van Leeuwen "Homogeneous Catalysis: Understanding the Art", 2004, Wiley-VCH, Weinheim. :RCH=CH2 + HCN -> RCH2-CH2-CN Stoichiometry and mechanism The reaction involves the addition of and cyanide () to the substrate. Usually the substrate is an alkene and the product is a nitrile. The reaction proceeds via the oxidative addition of HCN to a low-valent metal complex to give a hydrido cyanide complex. Subsequent binding of the alkene gives the intermediate , which then undergoes migratory insertion to give an alkylmetal cya ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature. Structure and general properties Hydrogen cyanide is a linear molecule, with a triple bond between carbon and nitrogen. The tautomer of HCN is HNC, hydrogen isocyanide. Hydrogen cyanide is weakly acidic with a p''K''a of 9.2. It partially ionizes in water solution to give the cyanide anion, CN−. A solution of hydrogen cyanide in water, represented as HCN, is called ''hydrocyanic acid''. The salts of the cyanide ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylamine
Methylamine is an organic compound with a formula of . This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine. Methylamine is sold as a solution in methanol, ethanol, tetrahydrofuran, or water, or as the anhydrous gas in pressurized metal containers. Industrially, methylamine is transported in its anhydrous form in pressurized railcars and tank trailers. It has a strong odor similar to rotten fish. Methylamine is used as a building block for the synthesis of numerous other commercially available compounds. Industrial production Methylamine is prepared commercially by the reaction of ammonia with methanol in the presence of an aluminosilicate catalyst. Dimethylamine and trimethylamine are co-produced; the reaction kinetics and reactant ratios determine the ratio of the three products. The product most favored by the reaction kinetics is trimethylamine. : In this way, an estimated 115,000 tons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


2CH)2AlCN)3-2D.png)