|
Coarctate Reaction
In the classification of organic reactions by transition state topology, a coarctate reaction (from L. ''coarctare'' "to constrict") is a third, comparatively uncommon topology, after linear topology and pericyclic topology (itself subdivided into Hückel and Möbius topologies). Transition state topologies Reactions of linear topology are the most common, and consist of all transformations whose transition states are acyclic, including addition, elimination, substitution, and (some types of) fragmentation reactions. In contrast, in pericyclic reactions, the atoms under chemical change form a closed cycle, and include reactions like the Diels-Alder reaction and Cope rearrangement, among many others. In contrast to these types of reactions, a coarctate reaction is characterized by a doubly cyclic transition state, in which at least one atom undergoes the simultaneous making and breaking of two bonds. Thus, the topology of the transition state of a coarctate reaction is a constr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition State Topologies
Transition or transitional may refer to: Mathematics, science, and technology Biology * Transition (genetics), a point mutation that changes a purine nucleotide to another purine (A ↔ G) or a pyrimidine nucleotide to another pyrimidine (C ↔ T) * Transitional fossil, any fossilized remains of a lifeform that exhibits the characteristics of two distinct taxonomic groups * A phase during childbirth contractions during which the cervix completes its dilation Gender and sex * Gender transitioning, the process of changing one's gender presentation to accord with one's internal sense of one's gender – the idea of what it means to be a man or woman * Sex reassignment therapy, the physical aspect of a gender transition Physics * Phase transition, a transformation of the state of matter; for example, the change between a solid and a liquid, between liquid and gas or between gas and plasma * Quantum phase transition, a phase transformation between different quantum phases * Quantum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organic Photochemistry, photochemical reactions and organic redox reaction, redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition State
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked with the double dagger ‡ symbol. As an example, the transition state shown below occurs during the SN2 reaction of bromoethane with a hydroxide anion: The activated complex of a reaction can refer to either the transition state or to other states along the reaction coordinate between reactants and products, especially those close to the transition state.Peter Atkins and Julio de Paula, ''Physical Chemistry'' (8th ed., W.H. Freeman 2006), p.809 According to the transition state theory, once the reactants have passed through the transition state configuration, they always continue to form products. History of concept The concept of a transition state has been important in many theories of the rates at which chemical reactions occ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Topology
In mathematics, topology (from the Greek language, Greek words , and ) is concerned with the properties of a mathematical object, geometric object that are preserved under Continuous function, continuous Deformation theory, deformations, such as Stretch factor, stretching, Twist (mathematics), twisting, crumpling, and bending; that is, without closing holes, opening holes, tearing, gluing, or passing through itself. A topological space is a set (mathematics), set endowed with a structure, called a ''Topology (structure), topology'', which allows defining continuous deformation of subspaces, and, more generally, all kinds of continuity (mathematics), continuity. Euclidean spaces, and, more generally, metric spaces are examples of a topological space, as any distance or metric defines a topology. The deformations that are considered in topology are homeomorphisms and homotopy, homotopies. A property that is invariant under such deformations is a topological property. Basic exampl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linear Topology
In algebra, a linear topology on a left A-module M is a topology In mathematics, topology (from the Greek language, Greek words , and ) is concerned with the properties of a mathematical object, geometric object that are preserved under Continuous function, continuous Deformation theory, deformations, such ... on M that is invariant under translations and admits a fundamental system of neighborhood of 0 that consists of submodules of M. If there is such a topology, M is said to be linearly topologized. If A is given a discrete topology, then M becomes a topological A-module with respect to a linear topology. See also * * * * * * * * * References * Bourbaki, N. (1972). Commutative algebra (Vol. 8). Hermann. Topology Topological algebra Topological groups {{algebra-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pericyclic Reaction
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overlap in a continuous cycle at the transition state. Pericyclic reactions stand in contrast to ''linear reactions'', encompassing most organic transformations and proceeding through an acyclic transition state, on the one hand and '' coarctate reactions'', which proceed through a doubly cyclic, concerted transition state on the other hand. Pericyclic reactions are usually rearrangement or addition reactions. The major classes of pericyclic reactions are given in the table below (the three most important classes are shown in bold). Ene reactions and cheletropic reactions are often classed as group transfer reactions and cycloadditions/cycloeliminations, respectively, while dyotropic reactions and group transfer reactions (if ene reactions are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Möbius–Hückel Concept
In chemistry, the Möbius–Hückel treatment is a methodology used to predict whether a reaction is allowed or forbidden. It is often used alone with the Woodward–Hoffmann approach. The description in this article uses the plus-minus sign notation for parity as shorthand while proceeding around a cycle of orbitals in a molecule or system, while the Woodward–Hoffmann methodology uses a large number of rules with the same consequences. Introduction One year following the Woodward–Hoffmann and Longuet-Higgins–Abrahmson publications, it was noted by Zimmerman that both transition states and stable molecules sometimes involved a Möbius array of basis orbitals. The Möbius–Hückel treatment provides an alternative to the Woodward–Hoffmann one. In contrast to the Woodward–Hoffmann approach the Möbius–Hückel treatment is not dependent on symmetry and only requires counting the number of plus-minus sign inversions in proceeding around the cyclic array of orbitals. Wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Open-chain Compound
In chemistry, an open-chain compound (also spelled as open chain compound) or acyclic compound (Greek prefix "α", ''without'' and "κύκλος", ''cycle'') is a compound with a linear structure, rather than a cyclic one. An open-chain compound having no side chains is called a straight-chain compound (also spelled as straight chain compound). Many of the simple molecules of organic chemistry, such as the alkanes and alkenes, have both linear and ring isomers, that is, both acyclic and cyclic, with the latter often classified as aromatic. For those with 4 or more carbons, the linear forms can have straight-chain or branched-chain isomers. The lowercase prefix ''n-'' denotes the straight-chain isomer; for example, ''n''-butane is straight-chain butane, whereas ''i''-butane is isobutane. Cycloalkanes are isomers of alkenes, not of alkanes, because the ring's closure involves a C-C bond. Having no rings (aromatic or otherwise), all open-chain compounds are aliphatic. Typically in bioc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fragmentation Reaction
Fragmentation or fragmented may refer to: Computers * Fragmentation (computing), a phenomenon of computer storage * File system fragmentation, the tendency of a file system to lay out the contents of files non-continuously * Fragmented distribution attack, in computer security * IP fragmentation, a process in computer networking Science * Fragmentation (cell biology), in cells * Fragmentation (reproduction), a form of asexual reproduction * Fragmentation of memory, a psychological disorder * Fragmentation (mass spectrometry), a technique to study structure of molecules * Fragmentation (weaponry), a feature of explosive weaponry * Fragmentation (medicine), an operation that breaks of solid matter in a body part into pieces, such as kidney stones * Fragmentation, the quantification by photoanalysis of blasted material * Hadronization, with quarks Other * Fragmentation (economics), a process of globalization * Fragmentation (music), a compositional technique * Fragmentation (socio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diels–Alder Reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally-allowed +2cycloaddition with Woodward–Hoffmann symbol π4s_+_π2s.html" ;"title="sub>π4s + π2s">sub>π4s + π2s It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cope Rearrangement
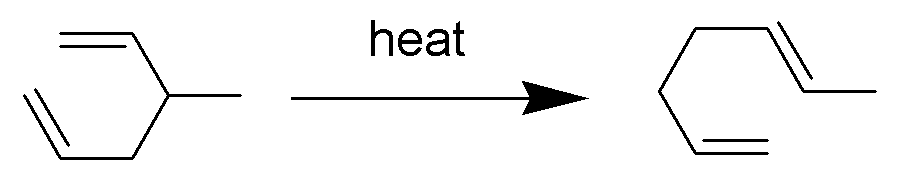
The Cope rearrangement is an extensively studied organic reaction involving the ,3sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope and Elizabeth Hardy. For example, 3-methyl-hexa-1,5-diene heated to 300 °C yields hepta-1,5-diene. The Cope rearrangement causes the fluxional states of the molecules in the bullvalene family. Mechanism The Cope rearrangement is the prototypical example of a concerted sigmatropic rearrangement. It is classified as a ,3sigmatropic rearrangement with the Woodward–Hoffmann symbol π2s+σ2s+π2s.html" ;"title="sub>π2s+σ2s+π2s">sub>π2s+σ2s+π2sand is therefore thermally allowed. It is sometimes useful to think of it as going through a transition state energetically and structurally equivalent to a diradical, although the diradical is not usually a true intermediate (potential energy minimum). The chair transition state illustrated here is preferred in open-chain systems (as shown by the Doering-Roth experiments ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyldioxirane
Dimethyldioxirane (DMDO), also referred to as Murray's reagent in reference to Robert W. Murray, is a dioxirane derived from acetone and can be considered as a monomer of acetone peroxide. It is a powerful yet selective oxidizing agent which finds use in organic synthesis. It is known only in the form of a dilute solution, usually in acetone, and hence the properties of the pure material are largely unknown. Synthesis DMDO is not commercially available because of its instability. DMDO can be prepared as dilute solutions (~0.1 M) by treatment of acetone with potassium peroxymonosulfate , usually in the form of Oxone (2KHSO5·KHSO4·K2SO4). : The preparation of DMDO is rather inefficient (typical yields < 3%) and typically only yields a relatively dilute solution in acetone (only up to approximately 0.1 M). This is tolerable as preparation uses inexpensive substances: |