|
Cinnamyl Acetate
Cinnamyl acetate (3-phenylprop-2-enyl acetate) is a chemical compound of the cinnamyl ester family, in which the variable R group is substituted by a methyl group. As a result of the non-aromatic carbon-carbon double bond, cinnamyl acetate can exist in a ''Z'' and an ''E'' configuration: Cinnamyl acetate naturally occurs in fresh bark of cinnamon (''Cinnamomum zeylanicum Blume'' and other Cinnamomum species), with concentrations of 2,800–51,000 ppm. Cinnamyl acetate is used as a flavour ester in for example bread and animal feed and has a sweet floral-fruity fragrance. Moreover, it is used in several cosmetics, some toiletries but also in non-cosmetic products, for example detergents. Legislation and control Cinnamyl acetate, used in fragrances and as flavour ingredient, has been discussed by several institutions. In 1965, the compound was annotated as 'Generally Recognized as Safe as a flavor ingredient’ by the Flavor and Extract Manufacturers" Association (FEMA). Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For example, protonation of methanol gives an elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transesterification
In organic chemistry, transesterification is the process of exchanging the organic group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. The reaction can also be accomplished with the help of other enzymes, particularly lipases (one example is the lipase E.C.3.1.1.3). Strong acids catalyse the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile, whereas bases catalyse the reaction by removing a proton from the alcohol, thus making it more nucleophilic. If the alcohol produced by the reaction can be separated from the reactants by distillation this will drive the equilibrium toward the products, this means that esters with larger alkoxy groups can be made from methyl or ethyl esters in high purity by heating the mixture of ester, acid/base, and large alcohol. Mechanism In the transesterification mechanism, the carbonyl carbon of the starting e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
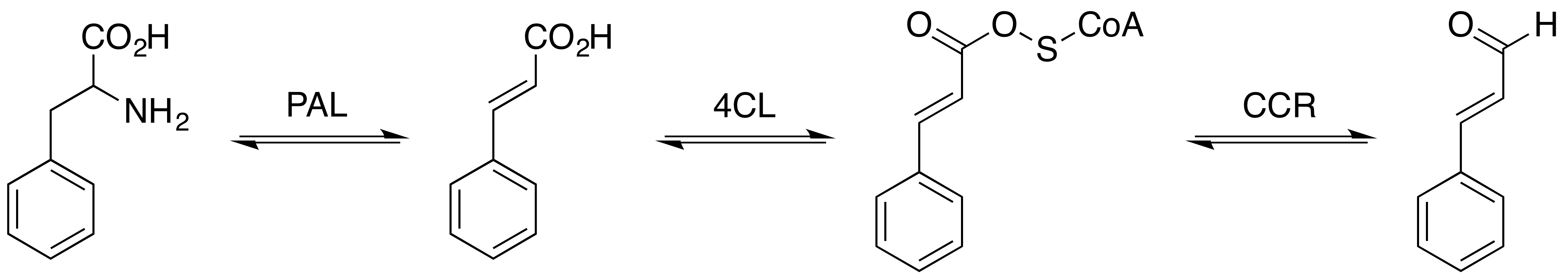
Cinnamaldehyde
Cinnamaldehyde is an organic compound with the formula(C9H8O) C6H5CH=CHCHO. Occurring naturally as predominantly the ''trans'' (''E'') isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus ''Cinnamomum''. The essential oil of cinnamon bark is about 90% cinnamaldehyde. Cinnamaldehyde decomposes to styrene because of oxidation as a result of bad storage or transport conditions. Styrene especially forms in high humidity and high temperatures. This is the reason why cinnamon contains small amounts of styrene. Structure and synthesis Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugène-Melchior Péligot and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854. The natural product is ''trans''-cinnamaldehyde. The molecule consists of a benzene rin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation, C=C (and other) bonds ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepatocyte
A hepatocyte is a cell of the main parenchymal tissue of the liver. Hepatocytes make up 80% of the liver's mass. These cells are involved in: * Protein synthesis * Protein storage * Transformation of carbohydrates * Synthesis of cholesterol, bile salts and phospholipids * Detoxification, modification, and excretion of exogenous and endogenous substances * Initiation of formation and secretion of bile Structure The typical hepatocyte is cubical with sides of 20-30 μm, (in comparison, a human hair has a diameter of 17 to 180 μm).The diameter of human hair ranges from 17 to 181 μm. The typical volume of a hepatocyte is 3.4 x 10−9 cm3. Smooth endoplasmic reticulum is abundant in hepatocytes, in contrast to most other cell types. Microanatomy Hepatocytes display an eosinophilic cytoplasm, reflecting numerous mitochondria, and basophilic stippling due to large amounts of smooth endoplasmic reticulum and free ribosomes. Brown lipofuscin granules are also observed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylesterase
The enzyme carboxylesterase (or carboxylic-ester hydrolase, EC 3.1.1.1; systematic name carboxylic-ester hydrolase) catalyzes reactions of the following form: :a carboxylic ester + H2O \rightleftharpoons an alcohol + a carboxylate Most enzymes from this group are serine hydrolases belonging to the superfamily of proteins with α/β hydrolase fold. Some exceptions include an esterase with β-lactamase-like structure (). Carboxylesterases are widely distributed in nature, and are common in mammalian liver. Many participate in phase I metabolism of xenobiotics such as toxins or drugs; the resulting carboxylates are then conjugated by other enzymes to increase solubility and eventually excreted. The essential polyunsaturated fatty acid arachidonic acid (AA C20 H32 O2; 20: 4, n-6), formed by the synthesis from dietary linoleic acid (LA: C18H32O2 18:2, n-6), has a role as a human carboxylesterase inhibitor. The carboxylesterase family of evolutionarily related proteins (those w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloramphenicol
Chloramphenicol is an antibiotic useful for the treatment of a number of bacterial infections. This includes use as an eye ointment to treat conjunctivitis. By mouth or by injection into a vein, it is used to treat meningitis, plague, cholera, and typhoid fever. Its use by mouth or by injection is only recommended when safer antibiotics cannot be used. Monitoring both blood levels of the medication and blood cell levels every two days is recommended during treatment. Common side effects include bone marrow suppression, nausea, and diarrhea. The bone marrow suppression may result in death. To reduce the risk of side effects treatment duration should be as short as possible. People with liver or kidney problems may need lower doses. In young children a condition known as gray baby syndrome may occur which results in a swollen stomach and low blood pressure. Its use near the end of pregnancy and during breastfeeding is typically not recommended. Chloramphenicol is a broad-spectrum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
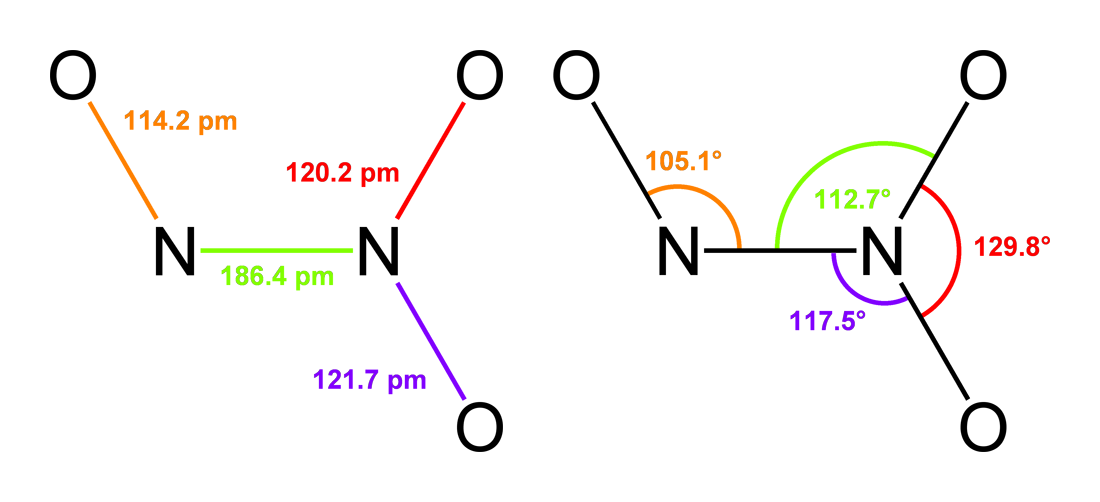
Dinitrogen Trioxide
Dinitrogen trioxide is the chemical compound with the formula N2O3. It is one of the simple nitrogen oxides. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F): :NO + NO2 N2O3 Dinitrogen trioxide is only isolable at low temperatures, i.e. in the liquid and solid phases. In liquid and solid states, it has a deep blue color. At higher temperatures the equilibrium favors the constituent gases, with ''K''diss = 193 kPa (25 °C). This compound is sometimes called "nitrogen trioxide", but this name properly refers to another compound, the (uncharged) nitrate radical . Structure and bonding Typically, N–N bonds are similar in length to that in hydrazine (145 pm). Dinitrogen trioxide, however, has an unusually long N–N bond at 186 pm. Some other nitrogen oxides also possess long N–N bonds, including dinitrogen tetroxide (175 pm). The N2O3 molecule is planar and exhibits Cs symmet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Addition Reaction
In organic chemistry, an addition reaction is, in simplest terms, an organic reaction where two or more molecules combine to form a larger one (the adduct).. Addition reactions are limited to chemical compounds that have multiple bonds, such as molecules with carbon–carbon double bonds (alkenes), or with triple bonds (alkynes), and compounds that have rings, which are also considered points of unsaturation. Molecules containing carbon— hetero double bonds like carbonyl () groups, or imine () groups, can undergo addition, as they too have double-bond character. An addition reaction is the reverse of an elimination reaction. For instance, the hydration of an alkene to an alcohol is reversed by dehydration. There are two main types of polar addition reactions: electrophilic addition and nucleophilic addition. Two non-polar addition reactions exist as well, called free-radical addition and cycloadditions. Addition reactions are also encountered in polymerizations and called a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase-transfer Catalyst
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the transition of a reactant from one phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of heterogeneous catalysis. Ionic reactants are often soluble in an aqueous phase but insoluble in an organic phase in the absence of the phase-transfer catalyst. The catalyst functions like a detergent for solubilizing the salts into the organic phase. Phase-transfer catalysis refers to the acceleration of the reaction upon the addition of the phase-transfer catalyst. By using a PTC process, one can achieve faster reactions, obtain higher conversions or yields, make fewer byproducts, eliminate the need for expensive or dangerous solvents that will dissolve all the reactants in one phase, eliminate the need for expensive raw materials and/or minimize waste problems. Phase-transfer catalysts are especially useful in green chemistry—by allowing the use of water, the nee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |