|
Cerlapirdine
Cerlapirdine (USAN; SAM-531, WAY-262,531, PF-05212365) is a drug which was under development by Wyeth/Pfizer for the treatment of cognitive disorders associated with Alzheimer's disease and schizophrenia. In a phase II clinical trial it demonstrated a trend toward efficacy along with a good side effect profile and no incidence of serious adverse events, but no further development has occurred since 2011. It exerts its effects by acting as a selective 5-HT6 receptor antagonist. See also * Idalopirdine * Latrepirdine Latrepirdine ( INN, also known as dimebolin and sold as Dimebon) is an antihistamine drug which has been used clinically in Russia since 1983. Research was conducted in both Russia and western nations into potential applications as a neuroprotec ... References {{Serotonergics Dimethylamino compounds Abandoned drugs 1-Naphthyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Latrepirdine
Latrepirdine (INN, also known as dimebolin and sold as Dimebon) is an antihistamine drug which has been used clinically in Russia since 1983. Research was conducted in both Russia and western nations into potential applications as a neuroprotective drug to treat Alzheimer's disease and, possibly, as a nootropic, as well. After a major phase III clinical trial for Alzheimer's disease (AD) treatment failed to show any benefit, three other AD trials continued.Novel Alzheimer's Drug Flops ''MedPage Today'', March 03, 2010 Major industry-based development in this indication essentially stopped after another Phase III trial suffered the same fate in 2012.Sweetlove M: Phase III CONCERT Trial of Latrepirdine. Negative results. Pharm Med 2012;26(2):113-115 Latrepirdine failed in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Idalopirdine
Idalopirdine (INN) (code names Lu AE58054,) is a potent and selective 5-HT6 receptor antagonist under development by Lundbeck as an augmentation therapy for the treatment of cognitive deficits associated with Alzheimer's disease and schizophrenia. As of October 2013 it is in phase III clinical trials. A phase III trial of two different daily doses of Lu AE58054 on top of 10 mg of donepezil for mild-to-moderate Alzheimer's failed to meet its primary endpoint Clinical endpoints or clinical outcomes are outcome measures referring to occurrence of disease, symptom, sign or laboratory abnormality constituting a target outcome in clinical research trials. The term may also refer to any disease or sign tha ... with either dose. Two further phase III trials failed too, the company confirmed in early 2017. See also * Cerlapirdine * Latrepirdine References External links Lundbeck expands its pipeline - initiating phase II clinical trials with a new compound for the treat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT6 Receptor
The 5HT6 receptor is a subtype of 5HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gs and mediates excitatory neurotransmission. ''HTR6'' denotes the human gene encoding for the receptor. Distribution The 5HT6 receptor is expressed almost exclusively in the brain. It is distributed in various areas including, but not limited to, the olfactory tubercle, cerebral cortex (frontal and entorhinal regions), nucleus accumbens, striatum, caudate nucleus, hippocampus, and the molecular layer of the cerebellum. Based on its abundance in extrapyramidal, limbic, and cortical regions it can be suggested that the 5HT6 receptor plays a role in functions like motor control, emotionality, cognition, and memory. Function Blockade of central 5HT6 receptors has been shown to increase glutamatergic and cholinergic neurotransmission in various brain areas, whereas activation enhances GA ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative name is muriate, derived from hydrochloric acid's ancient name: muriatic acid. Uses Converting amines into their hydrochlorides is a common way to improve their water solubility, which can be desirable for substances used in medications. The European Pharmacopoeia lists more than 200 hydrochlorides as active ingredients in medications. These hydrochlorides, compared to free bases, may more readily dissolve in the gastrointestinal tract and be absorbed into the bloodstream more quickly. Additionally, many hydrochlorides of amines have a longer shelf-life than their respective free bases. Amine hydrochlorides represent latent forms of a more reactive free base. In this regard, formation of an amine hydrochloride confers protection. This eff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
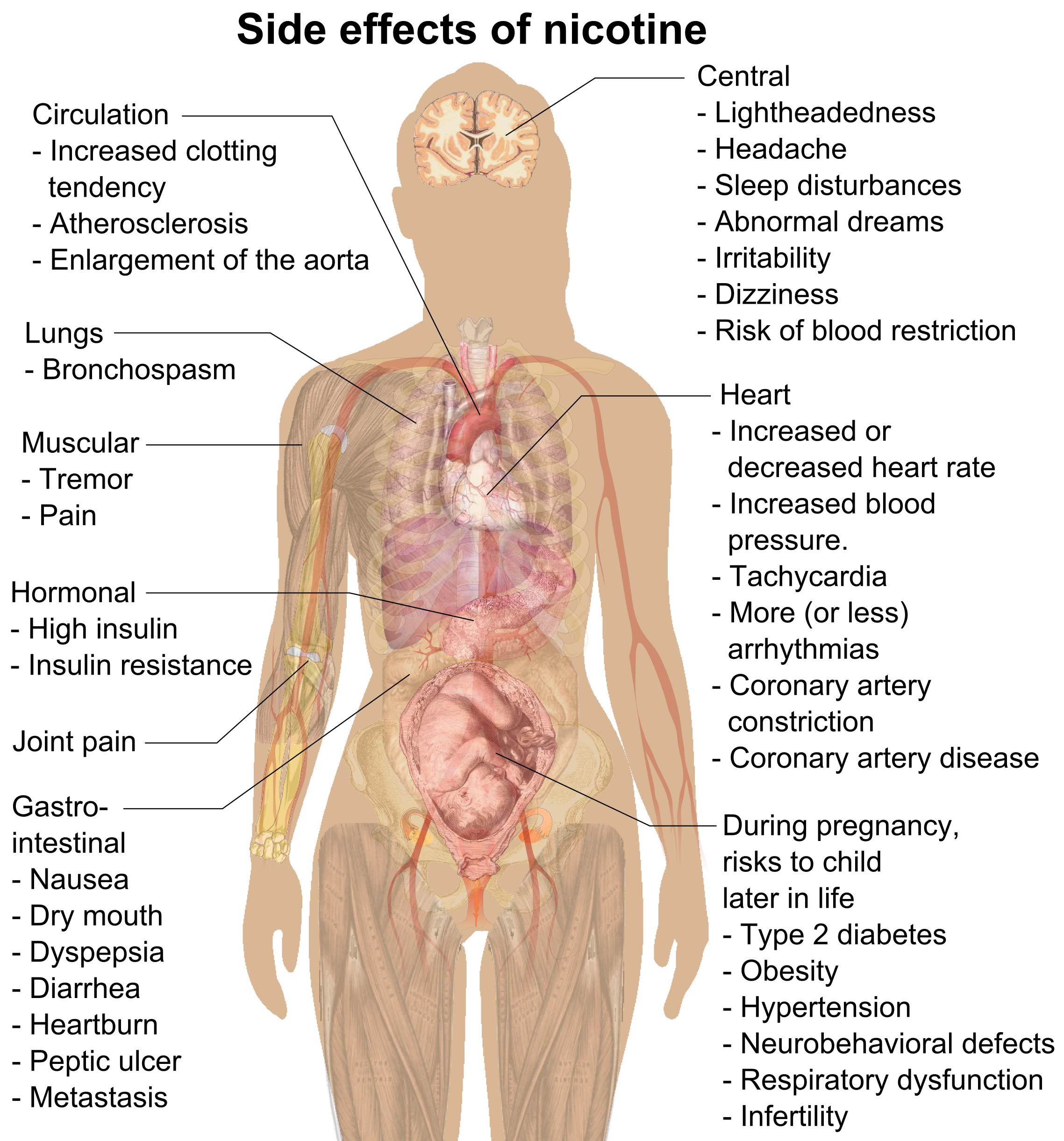
Side Effect
In medicine, a side effect is an effect, whether therapeutic or adverse, that is secondary to the one intended; although the term is predominantly employed to describe adverse effects, it can also apply to beneficial, but unintended, consequences of the use of a drug. Developing drugs is a complicated process, because no two people are exactly the same, so even drugs that have virtually no side effects, might be difficult for some people. Also, it is difficult to make a drug that targets one part of the body but that does not affect other parts, the fact that increases the risk of side effects in the untargeted parts. Occasionally, drugs are prescribed or procedures performed specifically for their side effects; in that case, said side effect ceases to be a side effect and is now an intended effect. For instance, X-rays were historically (and are currently) used as an imaging technique; the discovery of their oncolytic capability led to their employ in radiotherapy (ablation o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylamino Compounds
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005. Structure and synthesis The molecule consists of a nitrogen atom with two methyl substituents and one proton. Dimethylamine is a weak base and the pKa of the ammonium CH3--CH3 is 10.73, a value above methylamine (10.64) and trimethylamine (9.79). Dimethylamine reacts with acids to form salts, such as dimethylamine hydrochloride, an odorless white solid with a melting point of 171.5 °C. Dimethylamine is produced by catalytic reaction of methanol and ammonia at elevated temperatures and high pressure: :2 CH3OH + NH3 → (CH3)2NH + 2 H2O Natural occurrence Dimethylamine is found quite widely distributed in animals and plants, and is present in many foods at the level of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves " '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, |
Binding Selectivity
Binding selectivity is defined with respect to the binding of ligands to a substrate forming a complex. Binding selectivity describes how a ligand may bind more preferentially to one receptor than another. A selectivity coefficient is the equilibrium constant for the reaction of displacement by one ligand of another ligand in a complex with the substrate. Binding selectivity is of major importance in biochemistry and in chemical separation processes. Selectivity coefficient The concept of selectivity is used to quantify the extent to which one chemical substance, A, binds each of two other chemical substances, B and C. The simplest case is where the complexes formed have 1:1 stoichiometry. Then, the two interactions may be characterized by equilibrium constants ''K''AB and ''K''AC.The constant used here are ''association'' constants. ''Dissociation'' constants are used in some contexts. A dissociation constant is the reciprocal of an association constant. : + B AB; \mathit K_ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process – from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials – to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can vary i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United States Adopted Name
A United States Adopted Name (USAN) is a unique nonproprietary name assigned to a medication marketed in the United States. Each name is assigned by the USAN Council, which is co-sponsored by the American Medical Association (AMA), the United States Pharmacopeial Convention (USP), and the American Pharmacists Association (APhA). The USAN Program states that its goal is to select simple, informative, and unique nonproprietary names (also called generic names) for drugs by establishing logical nomenclature classifications based on pharmacological or chemical relationships. In addition to drugs, the USAN Council names agents for gene therapy and cell therapy, List of soft contact lens materials, contact lens polymers, surgical materials, diagnostics, carriers, and substances used as an excipient. The USAN Council works in conjunction with the World Health Organization (WHO) international nonproprietary name (INN) Expert Committee and national nomenclature groups to standardize drug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phases Of Clinical Research
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Summary Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory authority for use in the general population. Phase IV trials are 'post-marketing' or 'surveillance' studies conducted to monitor safety over sever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)