|
Frost
Frost is a thin layer of ice on a solid surface, which forms from water vapor that deposits onto a freezing surface. Frost forms when the air contains more water vapor than it can normally hold at a specific temperature. The process is similar to the formation of dew, except it occurs below the freezing point of water typically without crossing through a liquid state. Air always contains a certain amount of water vapor, depending on temperature. Warmer air can hold more than colder air. When the atmosphere contains more water than it can hold at a specific temperature, its relative humidity rises above 100% becoming supersaturated, and the excess water vapor is forced to deposit onto any nearby surface, forming seed crystals. The temperature at which frost will form is called the dew point, and depends on the humidity of the air. When the temperature of the air drops below its dew point, excess water vapor is forced out of solution, resulting in a phase change directly from wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wettability
Wetting is the ability of a liquid to displace gas to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. These interactions occur in the presence of either a gaseous phase or another liquid phase not miscible with the wetting liquid. The degree of wetting (wettability) is determined by a force balance between adhesive and cohesive forces. There are two types of wetting: non-reactive wetting and reactive wetting. Wetting is important in the bonding or adherence of two materials. The wetting power of a liquid, and surface forces which control wetting, are also responsible for related effects, including capillary effects. Surfactants can be used to increase the wetting power of liquids such as water. Wetting has gained increasing attention in nanotechnology and nanoscience research, following the development of nanomaterials over the past two decades (i.e., graphene, carbon nanotube, boron nitride nanomesh). E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deposition (phase Transition)
Deposition is the phase transition in which gas transforms into solid without passing through the liquid phase. Deposition is a thermodynamic process. The reverse of deposition is sublimation and hence sometimes deposition is called desublimation. Applications Examples One example of deposition is the process by which, in sub-freezing air, water vapour changes directly to ice without first becoming a liquid Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to th .... This is how frost and hoar frost form on the ground or other surfaces, including leaves. For deposition to occur, thermal energy must be removed from a gas. When the air becomes cold enough, water vapour in the air surrounding a leaf loses enough thermal energy to change into a solid. Even though the air tempera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Translucent
In the field of optics, transparency (also called pellucidity or diaphaneity) is the physical property of allowing light to pass through the material without appreciable light scattering by particles, scattering of light. On a macroscopic scale (one in which the dimensions are much larger than the wavelengths of the photons in question), the photons can be said to follow Snell's law. Translucency (also called translucence or translucidity) is the physical property of allowing light to pass through the material (with or without scattering of light). It allows light to pass through but the light does not necessarily follow Snell's law on the macroscopic scale; the photons may be scattered at either of the two interfaces, or internally, where there is a change in the index of refraction. In other words, a translucent material is made up of components with different indices of refraction. A transparent material is made up of components with a uniform index of refraction. Transparent m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Humidity
Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation (meteorology), precipitation, dew, or fog to be present. Humidity depends on the temperature and pressure of the system of interest. The same amount of water vapor results in higher relative humidity in cool air than warm air. A related parameter is the dew point. The amount of water vapor needed to achieve saturation increases as the temperature increases. As the temperature of a parcel of air decreases it will eventually reach the saturation point without adding or losing water mass. The amount of water vapor contained within a parcel of air can vary significantly. For example, a parcel of air near saturation may contain 8 g of water per cubic metre of air at , and 28 g of water per cubic metre of air at Three primary measurements of humidity are widely employed: abso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
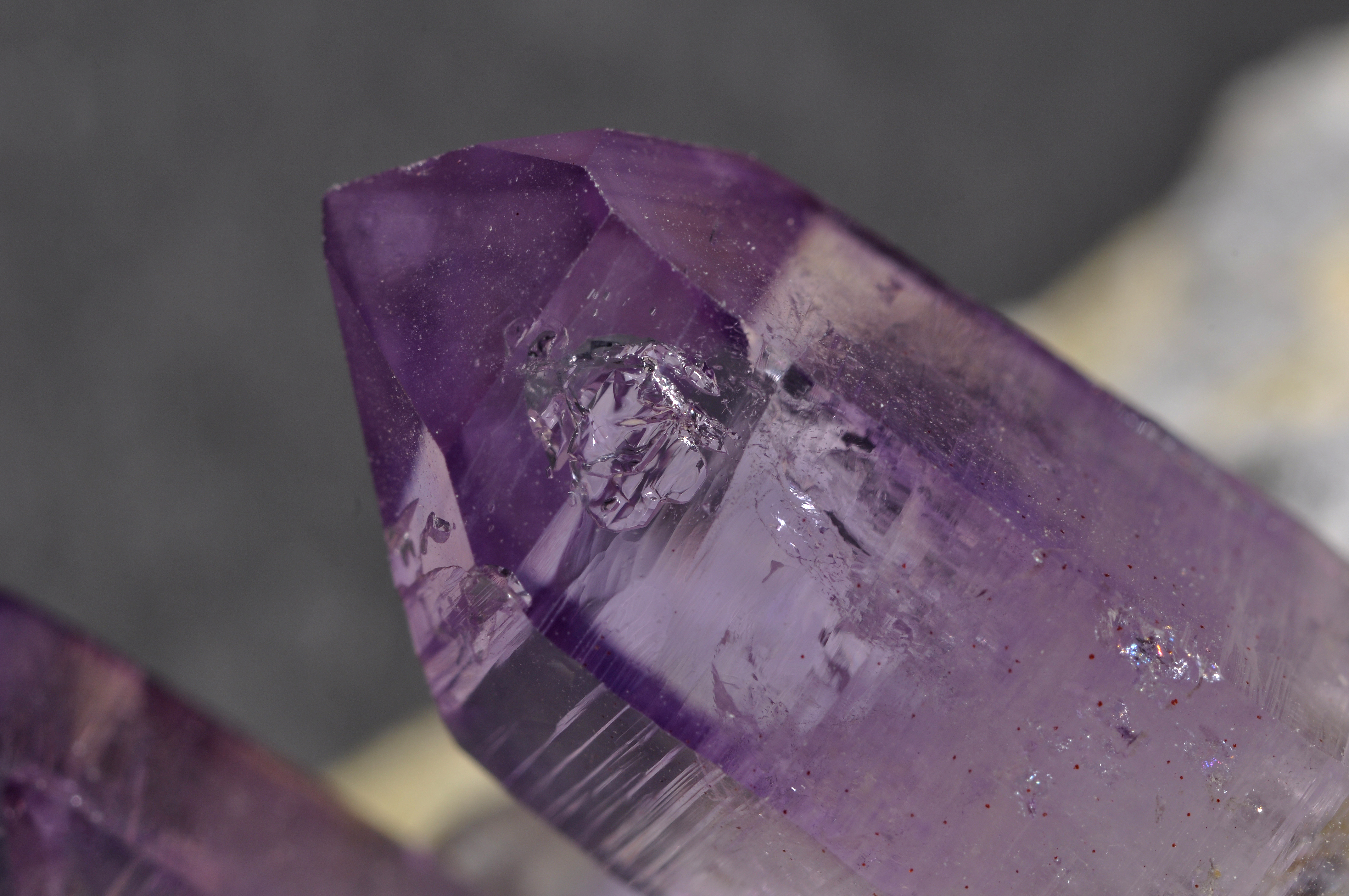
Frost Crystals
Ice crystals are solid water (known as ice) in symmetrical shapes including hexagonal columns, hexagonal plates, and dendritic crystals. Ice crystals are responsible for various atmospheric optical displays and cloud formations. Formation At ambient temperature and pressure, water molecules have a V shape. The two hydrogen atoms bond to the oxygen atom at a 105° angle. Ice crystals have a hexagonal crystal lattice, meaning the water molecules arrange themselves into layered hexagons upon freezing. Slower crystal growth from colder and drier atmospheres produces more hexagonal symmetry. Depending on environmental temperature and humidity, ice crystals can develop from the initial hexagonal prism into many symmetric shapes. Possible shapes for ice crystals are columns, needles, plates and dendrites. Mixed patterns are also possible. The symmetric shapes are due to depositional growth, which is when ice forms directly from water vapor in the atmosphere. Small spaces in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fractal
In mathematics, a fractal is a Shape, geometric shape containing detailed structure at arbitrarily small scales, usually having a fractal dimension strictly exceeding the topological dimension. Many fractals appear similar at various scales, as illustrated in successive magnifications of the Mandelbrot set. This exhibition of similar patterns at increasingly smaller scales is called self-similarity, also known as expanding symmetry or unfolding symmetry; if this replication is exactly the same at every scale, as in the Menger sponge, the shape is called affine geometry, affine self-similar. Fractal geometry lies within the mathematical branch of measure theory. One way that fractals are different from finite geometric figures is how they Scaling (geometry), scale. Doubling the edge lengths of a filled polygon multiplies its area by four, which is two (the ratio of the new to the old side length) raised to the power of two (the conventional dimension of the filled polygon). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleation Of Ice
An ice nucleus, also known as an ice nucleating particle (INP), is a particle which acts as the nucleus for the formation of an ice crystal in the atmosphere. Ice nucleation mechanisms There are a number of mechanisms of ice nucleation in the atmosphere through which ice nuclei can catalyse the formation of ice particles. In the upper troposphere, water vapor can deposit directly onto solid particles. In clouds warmer than about where liquid water can persist in a supercooled state, ice nuclei can trigger droplets to freeze. Contact nucleation can occur if an ice nucleus collides with a supercooled droplet, but the more important mechanism of freezing is when an ice nucleus becomes immersed in a supercooled water droplet and then triggers freezing. In the absence of an ice nucleating particle, pure water droplets can persist in a supercooled state to temperatures approaching where they freeze homogeneously. There are several research groups that study ice nucleating propert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleation
In thermodynamics, nucleation is the first step in the formation of either a new Phase (matter), thermodynamic phase or Crystal structure, structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled (at atmospheric pressure) significantly below 0°C, it will tend to Freezing, freeze into ice, but volumes of water cooled only a few degrees below 0°C often stay completely free of ice for long periods (supercooling). At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures nucleation is fast, and ice crystals appear after little or no delay. Nucleation is a common mechanism which generates first-order phase transitions, and it is the start of the process of forming a new thermodynamic phase. In contrast, new phas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Springer Science & Business Media
Springer Science+Business Media, commonly known as Springer, is a German multinational publishing company of books, e-books and peer-reviewed journals in science, humanities, technical and medical (STM) publishing. Originally founded in 1842 in Berlin, it expanded internationally in the 1960s, and through mergers in the 1990s and a sale to venture capitalists it fused with Wolters Kluwer and eventually became part of Springer Nature in 2015. Springer has major offices in Berlin, Heidelberg, Dordrecht, and New York City. History Julius Springer founded Springer-Verlag in Berlin in 1842 and his son Ferdinand Springer grew it from a small firm of 4 employees into Germany's then second-largest academic publisher with 65 staff in 1872.Chronology ". Springer Science+Business Media. In 1964, Springer expanded its business internationally, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystals
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and " rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third category of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |