|
Benzylisoquinoline Alkaloids
Substitution of the heterocycle isoquinoline at the C1 position by a benzyl group provides 1‑benzylisoquinoline, the most widely examined of the numerous benzylisoquinoline structural isomers. The 1-benzylisoquinoline moiety can be identified within numerous compounds of pharmaceutical interest, such as moxaverine; but most notably it is found within the structures of a wide variety of plant natural products, collectively referred to as benzylisoquinoline alkaloids. This class is exemplified in part by the following compounds: papaverine, noscapine, codeine, morphine, apomorphine, berberine, tubocurarine. Biosynthesis (''S'')- Norcoclaurine (higenamine) has been identified as the central 1-benzyl-tetrahydro-isoquinoline precursor from which numerous complex biosynthetic pathways eventually emerge. These pathways collectively lead to the structurally disparate compounds comprising the broad classification of plant natural products referred to as benzylisoquinoline alkaloids (B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quinol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
(S)-norcoclaurine
Higenamine (norcoclaurine) is a chemical compound found in a variety of plants including ''Nandina domestica'' (fruit), ''Aconitum carmichaelii'' (root), ''Asarum heterotropioides'', '' Galium divaricatum'' (stem and vine), ''Annona squamosa'', and ''Nelumbo nucifera'' (lotus seeds). Higenamine is found as an ingredient in sports and weight loss dietary supplements sold in the US. The US Food and Drug Administration has received reports of adverse effects from higenamine-containing supplements since 2014, but higenamine's health risks remain poorly understood. Legality Higenamine, also known as norcoclaurine HCl, is legal to use within food supplements in the UK, EU, the USA and Canada. Its main use is within food supplements developed for weight management and sports supplements. Traditional formulations with higenamine have been used for thousands of years within Chinese medicine and come from a variety of sources including fruit and orchids. There are no studies comparing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indolizidine
Indolizidine is a heterocyclic chemical compound that forms the central core of the indolizidine alkaloids such as swainsonine and castanospermine. See also * Indole * Indolizine * Tryptophan * Tryptamine Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the f ... References Indolizidines {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morphinan
Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others. Structure Morphinan has a phenanthrene core structure with the ''A'' ring remaining aromatic and the ''B'' and ''C'' rings being saturated, and an additional nitrogen-containing, six-membered, saturated ring, the ''D'' ring, being attached to carbons 9 and 13 of the core, and with the nitrogen being at position 17 of the composite. Of the major naturally occurring opiates of the morphinan type—morphine, codeine and thebaine—thebaine has no therapeutic properties (it causes seizures in mammals), but it provides a low-cost feedstock for the industrial production of at least four semi-synthetic opiate agonists, including hydrocodone, hydromorphone, oxycodone and oxymorphone, and the opioid antagonist naloxone. Structure-activity relationship The physiological behavior of morphinans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
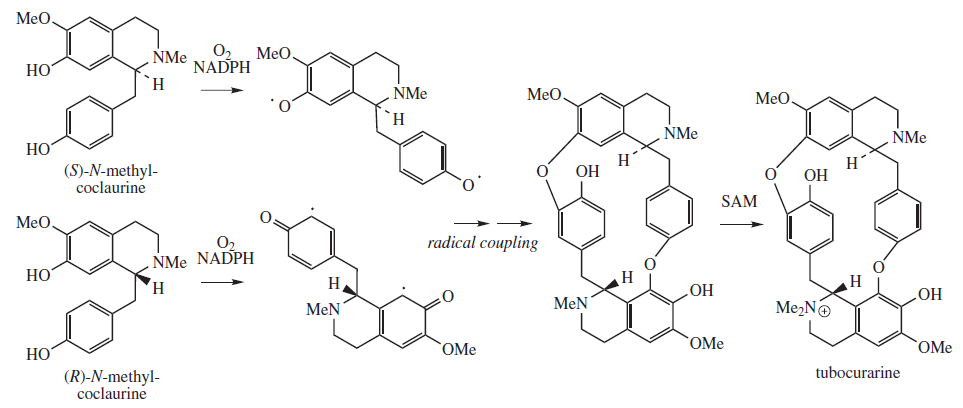
Tubocurarine
Tubocurarine (also known as ''d''-tubocurarine or DTC) is a toxic alkaloid historically known for its use as an arrow poison. In the mid-1900s, it was used in conjunction with an anesthetic to provide skeletal muscle relaxation during surgery or mechanical ventilation. It is now rarely used as an adjunct for clinical anesthesia because safer alternatives, such as cisatracurium and rocuronium, are available. History Tubocurarine is a naturally occurring mono-quaternary alkaloid obtained from the bark of the Menispermaceae, Menispermaceous South American plant ''Chondrodendron tomentosum'', a climbing vine known to the European world since the Spanish conquest of South America. Curare had been used as a source of arrow poison by South American natives to hunt animals, and they were able to eat the animals' contaminated flesh subsequently without any adverse effects because tubocurarine cannot easily cross mucous membranes. Thus, tubocurarine is effective only if given parenterally, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berberine
Berberine is a quaternary ammonium salt from the protoberberine group of benzylisoquinoline alkaloids found in such plants as ''Berberis vulgaris'' (barberry), ''Berberis aristata'' (tree turmeric), ''Mahonia aquifolium'' (Oregon grape), ''Hydrastis canadensis'' (goldenseal), ''Xanthorhiza simplicissima'' (yellowroot), ''Phellodendron amurense'' (Amur cork tree), ''Coptis chinensis'' (Chinese goldthread), ''Tinospora cordifolia'', ''Argemone mexicana'' (prickly poppy), and ''Eschscholzia californica'' (Californian poppy). Berberine is usually found in the roots, rhizomes, stems, and bark. Due to its yellow color, ''Berberis'' species were used to dye wool, leather, and wood. Under ultraviolet light, berberine shows a strong yellow fluorescence, making it useful in histology for staining heparin in mast cells. As a natural dye, berberine has a color index of 75160. Research and adverse effects The safety of using berberine for any condition is not adequately defined by high-qua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morphine
Morphine is a strong opiate that is found naturally in opium, a dark brown resin in poppies (''Papaver somniferum''). It is mainly used as a analgesic, pain medication, and is also commonly used recreational drug, recreationally, or to make other illicit drug, illicit opioids. There are numerous methods used to administer morphine: oral; sublingual administration, sublingual; via inhalation; intramuscular, injection into a muscle; by Subcutaneous injection, injection under the skin; intravenously; Intrathecally, injection into the space around the spinal cord; transdermal; or via rectal administration, rectal suppository. It acts directly on the central nervous system (CNS) to induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop with repeated administration. It can be taken for both acute pain and chronic pain and is frequently used for pain from myocardial infarction, kidney stones, and during Ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apomorphine
Apomorphine, sold under the brand name Apokyn among others, is a type of aporphine having activity as a non- selective dopamine agonist which activates both D2-like and, to a much lesser extent, D1-like receptors. It also acts as an antagonist of 5-HT2 and α-adrenergic receptors with high affinity. The compound is historically a morphine decomposition product made by boiling morphine with concentrated acid, hence the ''-morphine'' suffix. Contrary to its name, apomorphine does not actually contain morphine or its skeleton, nor does it bind to opioid receptors. The ''apo-'' prefix relates to it being a morphine derivative ("omesfrom morphine"). Historically, apomorphine has been tried for a variety of uses, including as a way to relieve anxiety and craving in alcoholics, an emetic (to induce vomiting), for treating stereotypies (repeated behaviour) in farmyard animals, and more recently in treating erectile dysfunction. Currently, apomorphine is used in the treatment of Pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Noscapine
Noscapine (also known as Narcotine, Nectodon, Nospen, Anarcotine and (archaic) Opiane) is a benzylisoquinoline alkaloid, of the phthalideisoquinoline structural subgroup, which has been isolated from numerous species of the family Papaveraceae (poppy family). It lacks significant hypnotic, euphoric, or analgesic effects affording it with very low addictive potential. This agent is primarily used for its antitussive (cough-suppressing) effects. Medical uses Noscapine is often used as an antitussive medication. A 2012 Dutch guideline, however, does not recommend its use for acute coughing. Side effects *Nausea *Vomiting *Loss of coordination *Hallucinations (auditory and visual) * Loss of sexual drive * Swelling of prostate *Loss of appetite *Dilated pupils *Increased heart rate * Shaking and muscle spasms *Chest pains *Increased alertness *Loss of any sleepiness *Loss of stereoscopic vision Interactions Noscapine can increase the effects of centrally sedating substances such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Papaverine
Papaverine (Latin ''papaver'', "poppy") is an opium alkaloid antispasmodic drug, used primarily in the treatment of visceral spasms and vasospasms (especially those involving the intestines, heart, or brain), occasionally in the treatment of erectile dysfunction and acute mesenteric ischemia. While it is found in the opium poppy, papaverine differs in both structure and pharmacological action from the analgesic morphine and its derivatives (such as codeine). History Papaverine was discovered in 1848 by Georg Merck (1825–1873). Merck was a student of the German chemists Justus von Liebig and August Hofmann, and he was the son of Emanuel Merck (1794–1855), founder of the Merck corporation, a major German chemical and pharmaceutical company. Uses Papaverine is approved to treat spasms of the gastrointestinal tract, bile ducts and ureter and for use as a cerebral and coronary vasodilator in subarachnoid hemorrhage (combined with balloon angioplasty) and coronary artery ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydropapaveroline
Tetrahydropapaveroline (norlaudanosoline) is a benzyltetrahydroisoquinoline alkaloid. It can be formed in trace amounts in the brain by a condensation reaction of dopamine and dopaldehyde (a metabolite of dopamine). It inhibits dopamine uptake within the cerebral cortex The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. The cerebral cortex mostly consists of the six-layered neocortex, with just 10% consistin .... References {{Reflist Dopamine reuptake inhibitors Tetrahydroisoquinolines Catechols ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |