|
Borromean Nucleus
A Borromean nucleus is an atomic nucleus comprising three bound components in which any subsystem of two components is unbound. This has the consequence that if one component is removed, the remaining two comprise an unbound resonance, so that the original nucleus is split into three parts. The name is derived from the Borromean rings, a system of three linked rings in which no pair of rings is linked. Examples of Borromean nuclei Many Borromean nuclei are light nuclei near the nuclear drip lines that have a nuclear halo and low nuclear binding energy. For example, the nuclei , , and each possess a two-neutron halo surrounding a core containing the remaining nucleons. These are Borromean nuclei because the removal of either neutron from the halo will result in a resonance unbound to one-neutron emission, whereas the dineutron (the particles in the halo) is itself an unbound system. Similarly, is a Borromean nucleus with a two-proton halo; both the diproton and are unbound. Add ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force. The diameter of the nucleus is in the range of () for hydrogen (the diameter of a single proton) to about for uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 26,634 (uranium atomic radiu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as or indicating a helium ion with a +2 charge (missing its two electrons). Once the ion gains electrons from its environment, the alpha particle becomes a normal (electrically neutral) helium atom . Alpha particles have a net spin of zero. Due to the mechanism of their production in standard alpha radioactive decay, alpha particles generally have a kinetic energy of about 5 MeV, and a velocity in the vicinity of 4% of the speed of light. (See discussion below for the limits of these figures in alpha decay.) They are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Three-body Force
A three-body force is a force that does not exist in a system of two objects but appears in a three-body system. In general, if the behaviour of a system of more than two objects cannot be described by the two-body interactions between all possible pairs, as a first approximation, the deviation is mainly due to a three-body force. The fundamental strong interaction does exhibit such behaviour, the most important example being the stability experimentally observed for the helium-3 isotope, which can be described as a 3-body quantum cluster entity of two protons and one neutron NPin stable superposition. Direct evidence of a 3-body force in helium-3 is known The existence of stable NPcluster calls into question models of the atomic nucleus that restrict nucleon interactions within shells to 2-body phenomenon. The three-nucleon-interaction is fundamentally possible because gluons, the mediators of the strong interaction, can couple to themselves. In particle physics, the interac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Efimov State
The Efimov effect is an effect in the quantum mechanics of few-body systems predicted by the Russian theoretical physicist V. N. Efimov in 1970. Efimov’s effect is where three identical bosons interact, with the prediction of an infinite series of excited three-body energy levels when a two-body state is exactly at the dissociation threshold. One corollary is that there exist bound states (called Efimov states) of three bosons even if the two-particle attraction is too weak to allow two bosons to form a pair. A (three-particle) Efimov state, where the (two-body) sub-systems are unbound, is often depicted symbolically by the Borromean rings. This means that if one of the particles is removed, the remaining two fall apart. In this case, the Efimov state is also called a Borromean state. Theory Efimov predicted that, as the pair interactions among three identical bosons approach resonance—that is, as the binding energy of some two-body bound state approaches zero or the scatteri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
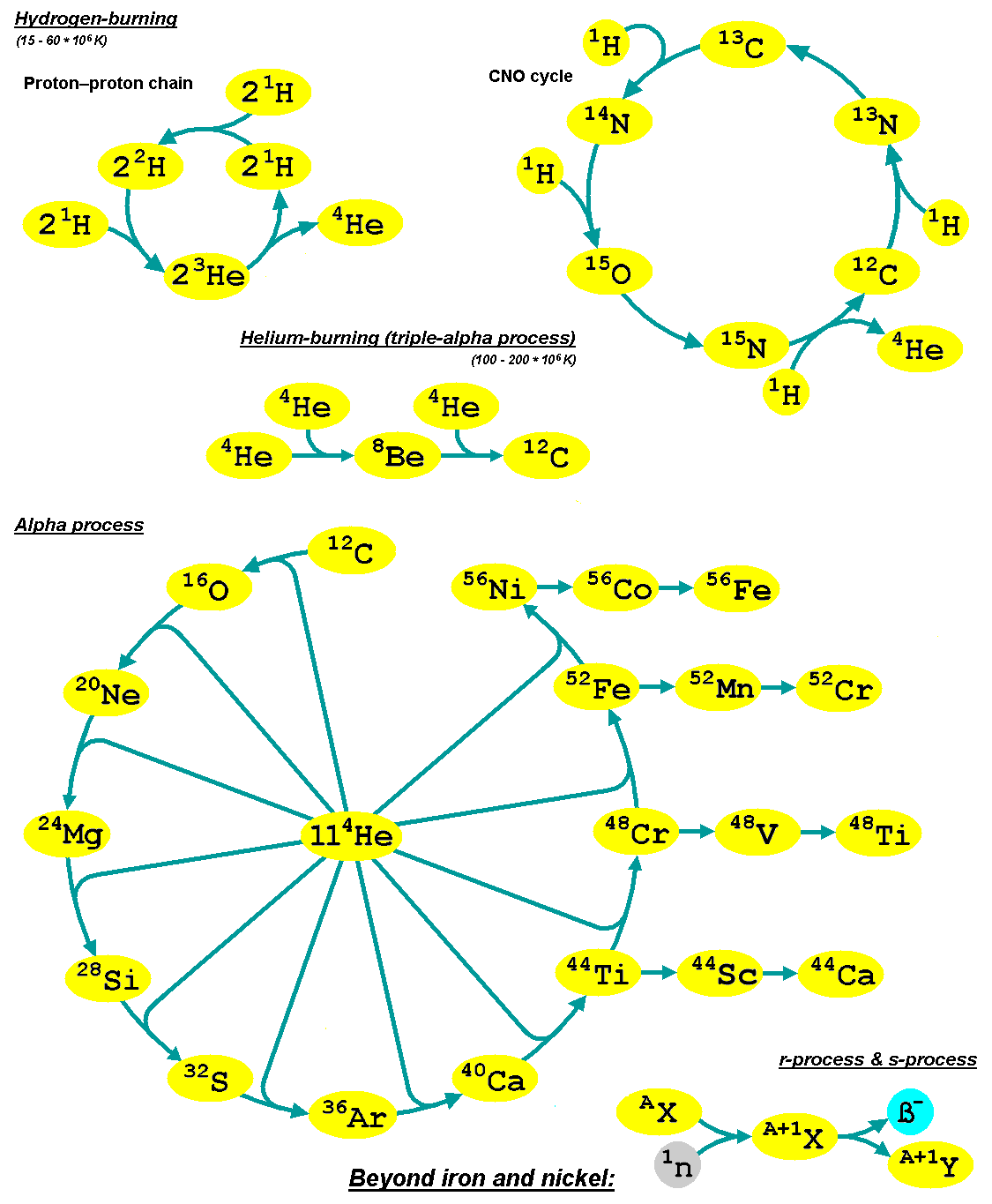
Alpha Process
The alpha process, also known as the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements, the other being the triple-alpha process. The triple-alpha process consumes only helium, and produces carbon. After enough carbon has accumulated, further reactions below take place, listed below. Each step only consumes helium and the product of the previous reaction. :\begin \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \end The energy produced each the reaction, , is primarily in the gamma ray (), with a small amount taken by the byproduct element, as added momentum. It is a common misconception that the above sequence ends at \, _^\mathrm \, (or \, _^\mathrm \,, which is a decay product of \, _^\mathrm \,) because it is the most tightly bound nuclide - i.e., having the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclides
A nuclide (or nucleide, from nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state. The word ''nuclide'' was coined by Truman P. Kohman in 1947. Kohman defined ''nuclide'' as a "species of atom characterized by the constitution of its nucleus" containing a certain number of neutrons and protons. The term thus originally focused on the nucleus. Nuclides vs isotopes A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon-13 with 6 protons and 7 neutrons. The nuclide concept (referring to individual nuclear species) emphasizes nuclear properties over chemical properties, while the isotope concept (grouping all atoms of each element) emphasizes chemical over nuclear. The neutron number has large effects on nuclear properties, but its effect on chemical reactions is negligible for most elements. Even in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triple-alpha Process
The triple-alpha process is a set of nuclear fusion reactions by which three helium-4 nuclei (alpha particles) are transformed into carbon. Triple-alpha process in stars Helium accumulates in the cores of stars as a result of the proton–proton chain reaction and the carbon–nitrogen–oxygen cycle. Nuclear fusion reaction of two helium-4 nuclei produces beryllium-8, which is highly unstable, and decays back into smaller nuclei with a half-life of , unless within that time a third alpha particle fuses with the beryllium-8 nucleus to produce an excited resonance state of carbon-12, called the Hoyle state, which nearly always decays back into three alpha particles, but once in about 2421.3 times releases energy and changes into the stable base form of carbon-12. When a star runs out of hydrogen to fuse in its core, it begins to contract and heat up. If the central temperature rises to 108 K, six times hotter than the Sun's core, alpha particles can fuse fast enough to get pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Astrophysics
Nuclear astrophysics is an interdisciplinary part of both nuclear physics and astrophysics, involving close collaboration among researchers in various subfields of each of these fields. This includes, notably, nuclear reactions and their rates as they occur in cosmic environments, and modeling of astrophysical objects where these nuclear reactions may occur, but also considerations of cosmic evolution of isotopic and elemental composition (often called chemical evolution). Constraints from observations involve multiple messengers, all across the electromagnetic spectrum ( nuclear gamma-rays, X-rays, optical, and radio/sub-mm astronomy), as well as isotopic measurements of solar-system materials such as meteorites and their stardust inclusions, cosmic rays, material deposits on Earth and Moon). Nuclear physics experiments address stability (i.e., lifetimes and masses) for atomic nuclei well beyond the regime of stable nuclides into the realm of radioactive/unstable nuclei, almost t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Excited State
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to an increase in energy level above a chosen starting point, usually the ground state, but sometimes an already excited state. The temperature of a group of particles is indicative of the level of excitation (with the notable exception of systems that exhibit negative temperature). The lifetime of a system in an excited state is usually short: spontaneous or induced emission of a quantum of energy (such as a photon or a phonon) usually occurs shortly after the system is promoted to the excited state, returning the system to a state with lower energy (a less excited state or the ground state). This return to a lower energy level is often loosely described as decay and is the inverse of excitation. Long-lived excited states are often called ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hoyle State
Carbon-12 (12C) is the most abundant of the two stable isotopes of carbon (carbon-13 being the other), amounting to 98.93% of element carbon on Earth; its abundance is due to the triple-alpha process by which it is created in stars. Carbon-12 is of particular importance in its use as the standard from which atomic masses of all nuclides are measured, thus, its atomic mass is exactly 12 daltons by definition. Carbon-12 is composed of 6 protons, 6 neutrons, and 6 electrons. History Before 1959, both the IUPAP and IUPAC used oxygen to define the mole; the chemists defining the mole as the number of atoms of oxygen which had mass 16 g, the physicists using a similar definition but with the oxygen-16 isotope only. The two organizations agreed in 1959/60 to define the mole as follows. ''Mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 12 gram of carbon 12; its symbol is "mol".'' This was adopted by the CIPM (Inter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diproton
Although there are nine known isotopes of helium (2He) (standard atomic weight: ), only helium-3 () and helium-4 () are stable. All radioisotopes are short-lived, the longest-lived being with a half-life of . The least stable is , with a half-life of (), although it is possible that may have an even shorter half-life. In the Earth's atmosphere, the ratio of to is . However, the isotopic abundance of helium varies greatly depending on its origin. In the Local Interstellar Cloud, the proportion of to is , which is times higher than that of atmospheric helium. Rocks from the Earth's crust have isotope ratios varying by as much as a factor of ten; this is used in geology to investigate the origin of rocks and the composition of the Earth's mantle. The different formation processes of the two stable isotopes of helium produce the differing isotope abundances. Equal mixtures of liquid and below separate into two immiscible phases due to differences in quantum statistics: at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resonance (particle Physics)
In particle physics, a resonance is the peak located around a certain energy found in differential cross sections of scattering experiments. These peaks are associated with subatomic particles, which include a variety of bosons, quarks and hadrons (such as nucleons, delta baryons or upsilon mesons) and their excitations. In common usage, "resonance" only describes particles with very short lifetimes, mostly high-energy hadrons existing for or less. The width of the resonance (''Γ'') is related to the mean lifetime (''τ'') of the particle (or its excited state) by the relation :\Gamma=\frac where ''h'' is the Planck constant and =\frac. Thus, the lifetime of a particle is the direct inverse of the particle's resonance width. For example, the charged pion has the second-longest lifetime of any meson, at . Therefore, its resonance width is very small, about or about 6.11 MHz. Pions are generally not considered as "resonances". The charged rho meson has a very short lifetime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |