|
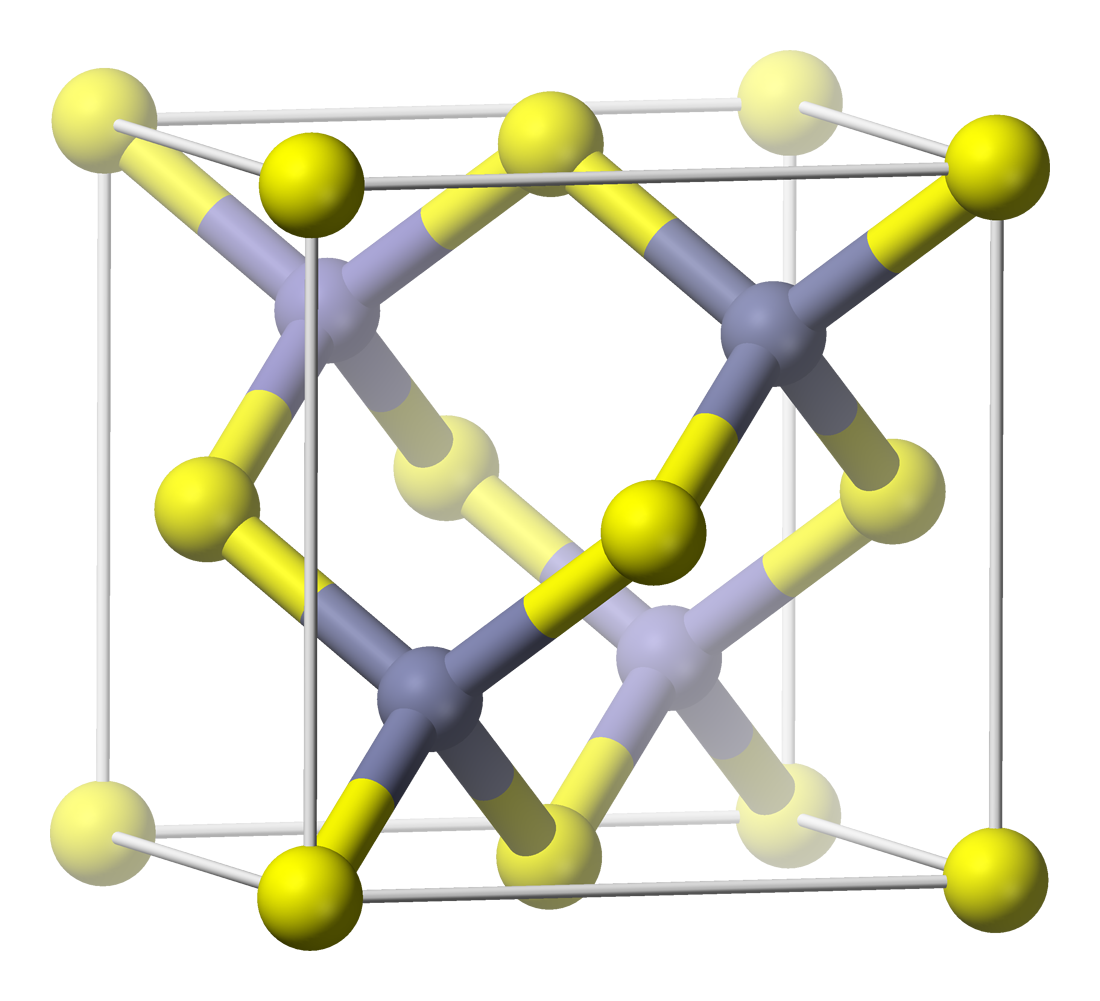
Boron Arsenide
Boron arsenide is a chemical compound involving boron and arsenic, usually with a chemical formula BAs. Other boron arsenide compounds are known, such as the subarsenide . Chemical synthesis of cubic BAs is very challenging and its single crystal forms usually have defects. Properties BAs is a cubic (sphalerite) semiconductor in the III-V family with a lattice constant of 0.4777 nm and an indirect band gap of 1.82 eV. Cubic BAs is reported to decompose to the subarsenide B12As2 at temperatures above 920 °C. Boron arsenide has a melting point of 2076 °C. The thermal conductivity of BAs is very high: around 1300 W/(m·K) at 300 K. The basic physical properties of cubic BAs have been experimentally measured: Band gap (1.82 eV), optical refractive index (3.29 at wavelength 657 nm), elastic modulus (326 GPa), shear modulus, Poisson's ratio, thermal expansion coefficient (3.85×10−6/K), and heat capacity. It can be alloyed with gallium arsenide to produce ternary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sphalerite
Sphalerite (sometimes spelled sphaelerite) is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in Sedimentary exhalative deposits, sedimentary exhalative, Carbonate-hosted lead-zinc ore deposits, Mississippi-Valley type, and Volcanogenic massive sulfide ore deposit, volcanogenic massive sulfide deposits. It is found in association with galena, chalcopyrite, pyrite (and other sulfide mineral, sulfides), calcite, dolomite (mineral), dolomite, quartz, rhodochrosite, and fluorite. German geologist Ernst Friedrich Glocker discovered sphalerite in 1847, naming it based on the Greek word ''sphaleros'', meaning "deceiving", due to the difficulty of identifying the mineral. In addition to zinc, sphalerite is an ore of cadmium, gallium, germanium, and indium. Miners have been known to refer to sphalerite as ''zinc blende'', ''black-jack'', and ''ruby blende''. Marmatite is an opaque ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium Arsenide
Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a Zincblende (crystal structure), zinc blende crystal structure. Gallium arsenide is used in the manufacture of devices such as microwave frequency integrated circuits, monolithic microwave integrated circuits, infrared light-emitting diodes, laser diodes, solar cells and optical windows. GaAs is often used as a substrate material for the epitaxial growth of other III-V semiconductors, including indium gallium arsenide, aluminum gallium arsenide and others. Preparation and chemistry In the compound, gallium has a +3 oxidation state. Gallium arsenide single crystals can be prepared by three industrial processes: * The vertical gradient freeze (VGF) process. * Crystal growth using a horizontal zone furnace in the Bridgman-Stockbarger technique, in which gallium and arsenic vapors react, and free molecules deposit on a seed crystal at the cooler end of the furnace. * Liquid encapsulated Czochralski process, Czoch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
III-V Compounds
Semiconductor materials are nominally small band gap insulators. The defining property of a semiconductor material is that it can be compromised by doping it with impurities that alter its electronic properties in a controllable way. Because of their application in the computer and photovoltaic industry—in devices such as transistors, lasers, and solar cells—the search for new semiconductor materials and the improvement of existing materials is an important field of study in materials science. Most commonly used semiconductor materials are crystalline inorganic solids. These materials are classified according to the periodic table groups of their constituent atoms. Different semiconductor materials differ in their properties. Thus, in comparison with silicon, compound semiconductors have both advantages and disadvantages. For example, gallium arsenide (GaAs) has six times higher electron mobility than silicon, which allows faster operation; wider band gap, which allo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Compounds
Boron compounds are compounds containing the element boron. In the most familiar compounds, boron has the formal oxidation state +3. These include oxides, sulfides, nitrides, and halides. Halides The trihalides adopt a planar trigonal structure. These compounds are Lewis acids in that they readily form adducts with electron-pair donors, which are called Lewis bases. For example, fluoride (F−) and boron trifluoride (BF3) combined to give the tetrafluoroborate anion, BF4−. Boron trifluoride is used in the petrochemical industry as a catalyst. The halides react with water to form boric acid. Boron is found in nature on Earth almost entirely as various oxides of B(III), often associated with other elements. More than one hundred borate minerals contain boron in oxidation state +3. These minerals resemble silicates in some respect, although boron is often found not only in a tetrahedral coordination with oxygen, but also in a trigonal planar configuration. Unlike silicates, b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to prepare it and characterize it in pure form. Its oxides form a family of anions known as silicates. Its melting and boiling points of 1414 °C and 3265 °C, respectively, are the second highest among all the metalloids and nonmetals, being surpassed only by boron. Silicon is the eighth most common element in the universe by mass, but very rarely occurs as the pure element in the Earth's crust. It is widely distributed in space in cosmic dusts, planetoids, and planets as various forms of silicon dioxide ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa. Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal conductivity. For instance, metals typically have high thermal conductivity and are very efficient at conducting heat, while the opposite is true for insulating materials like Rockwool or Styrofoam. Correspondingly, materials of high thermal conductivity are widely used in heat sink applications, and materials of low thermal conductivity are used as thermal insulation. The reciprocal of thermal conductivity is called thermal resistivity. The defining equation for thermal conductivity is \mathbf = - k \nabla T, where \mathbf is the heat flux, k is the thermal conductivity, and \nabla T is the temperature gradient. This is known as Fourier's Law for heat conduction. Although commonly expressed as a scalar, the most general form of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High-electron-mobility Transistor
A high-electron-mobility transistor (HEMT), also known as heterostructure FET (HFET) or modulation-doped FET (MODFET), is a field-effect transistor incorporating a junction between two materials with different band gaps (i.e. a heterojunction) as the channel instead of a doped region (as is generally the case for a MOSFET). A commonly used material combination is GaAs with AlGaAs, though there is wide variation, dependent on the application of the device. Devices incorporating more indium generally show better high-frequency performance, while in recent years, gallium nitride HEMTs have attracted attention due to their high-power performance. Like other FETs, HEMTs are used in integrated circuits as digital on-off switches. FETs can also be used as amplifiers for large amounts of current using a small voltage as a control signal. Both of these uses are made possible by the FET’s unique current–voltage characteristics. HEMT transistors are able to operate at higher frequenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium Nitride
Gallium nitride () is a binary III/ V direct bandgap semiconductor commonly used in blue light-emitting diodes since the 1990s. The compound is a very hard material that has a Wurtzite crystal structure. Its wide band gap of 3.4 eV affords it special properties for applications in optoelectronic, high-power and high-frequency devices. For example, GaN is the substrate which makes violet (405 nm) laser diodes possible, without requiring nonlinear optical frequency-doubling. Its sensitivity to ionizing radiation is low (like other group III nitrides), making it a suitable material for solar cell arrays for satellites. Military and space applications could also benefit as devices have shown stability in high radiation environments. Because GaN transistors can operate at much higher temperatures and work at much higher voltages than gallium arsenide (GaAs) transistors, they make ideal power amplifiers at microwave frequencies. In addition, GaN offers promising characteris ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Cell
A solar cell, or photovoltaic cell, is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon.Solar Cells chemistryexplained.com It is a form of photoelectric cell, defined as a device whose electrical characteristics, such as , , or resistance, vary when exposed to light. Individual solar cell devices are often the electrical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Carbide
Silicon carbide (SiC), also known as carborundum (), is a hard chemical compound containing silicon and carbon. A semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder and crystal since 1893 for use as an abrasive. Grains of silicon carbide can be bonded together by sintering to form very hard ceramics that are widely used in applications requiring high endurance, such as car brakes, car clutches and ceramic plates in bulletproof vests. Large single crystals of silicon carbide can be grown by the Lely method and they can be cut into gems known as synthetic moissanite. Electronic applications of silicon carbide such as light-emitting diodes (LEDs) and Cat's whisker detector, detectors in early radios were first demonstrated around 1907. SiC is used in semiconductor electronics devices that operate at high temperatures or high voltages, or both. Natural occurrence Naturally occurring moissanite is found in only minut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substrate (chemistry)
In chemistry, the term substrate is highly context-dependent. Broadly speaking, it can refer either to a chemical species being observed in a chemical reaction, or to a surface on which other chemical reactions or microscopy are performed. In the former sense, a reagent is added to the ''substrate'' to generate a product through a chemical reaction. The term is used in a similar sense in synthetic and organic chemistry, where the substrate is the chemical of interest that is being modified. In biochemistry, an enzyme substrate is the material upon which an enzyme acts. When referring to Le Chatelier's principle, the substrate is the reagent whose concentration is changed. ;Spontaneous reaction : :*Where S is substrate and P is product. ;Catalysed reaction : :*Where S is substrate, P is product and C is catalyst. In the latter sense, it may refer to a surface on which other chemical reactions are performed or play a supporting role in a variety of spectroscopic and microsco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |