|
Bohr Model
In atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity. It came after the solar system Joseph Larmor model (1897), the solar system Jean Perrin model (1901), the cubical model (1902), the Hantaro Nagaoka Saturnian model (1904), the plum pudding model (1904), the quantum Arthur Haas model (1910), the Rutherford model (1911), and the nuclear quantum John William Nicholson model (1912). The improvement over the 1911 Rutherford model mainly concerned the new quantum physical interpretation introduced by Haas and Nicholson, but forsaking any attempt to align with classical physics radiation. The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bohr Atom Model
In atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity. It came after the solar system Joseph Larmor model (1897), the solar system Jean Perrin model (1901), the cubical model (1902), the Hantaro Nagaoka Saturnian model (1904), the plum pudding model (1904), the quantum Arthur Haas model (1910), the Rutherford model (1911), and the nuclear quantum John William Nicholson model (1912). The improvement over the 1911 Rutherford model mainly concerned the new quantum physical interpretation introduced by Haas and Nicholson, but forsaking any attempt to align with classical physics radiation. The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Spectral Series
The emission spectrum of atomic hydrogen has been divided into a number of spectral series, with wavelengths given by the Rydberg formula. These observed spectral lines are due to the electron making transitions between two energy levels in an atom. The classification of the series by the Rydberg formula was important in the development of quantum mechanics. The spectral series are important in astronomical spectroscopy for detecting the presence of hydrogen and calculating red shifts. Physics A hydrogen atom consists of an electron orbiting its nucleus. The electromagnetic force between the electron and the nuclear proton leads to a set of quantum states for the electron, each with its own energy. These states were visualized by the Bohr model of the hydrogen atom as being distinct orbits around the nucleus. Each energy level, or electron shell, or orbit, is designated by an integer, as shown in the figure. The Bohr model was later replaced by quantum mechanics in which the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
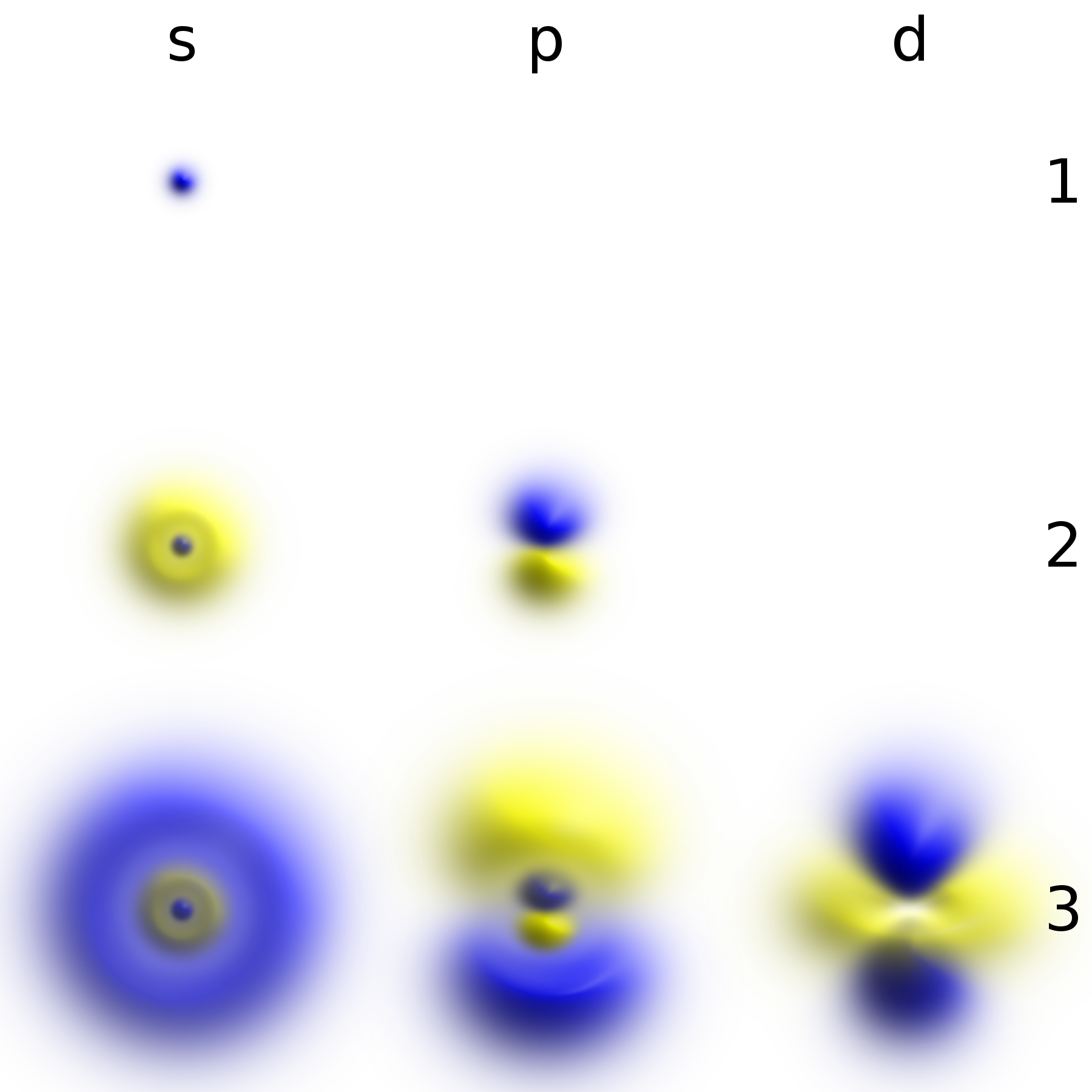
Atome Bohr Couches Electroniques KLM
Atome may refer to: *Atomè, Benin * Atôme, Angola *The , a two-piece swimsuit invented in 1932 by Jacques Heim Jacques Heim (8 May 1899 – 8 January 1967) was a French fashion designer and costume designer for theater and film, and was a manufacturer of women's furs. From 1930 to his death in 1967, he ran the fashion house (''maison de couture'') ''Ja ... See also * Atoma (other) * Tafi Atome Monkey Sanctuary, Ghana {{Disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Old Quantum Theory
The old quantum theory is a collection of results from the years 1900–1925 which predate modern quantum mechanics. The theory was never complete or self-consistent, but was rather a set of heuristic corrections to classical mechanics. The theory is now understood as the semi-classical approximation to modern quantum mechanics. The main and final accomplishments of the old quantum theory were the determination of the modern form of the periodic table by Edmund Stoner and the Pauli Exclusion Principle which were both premised on the Arnold Sommerfeld enhancements to the Bohr model of the atom. The main tool of the old quantum theory was the Bohr–Sommerfeld quantization condition, a procedure for selecting out certain states of a classical system as allowed states: the system can then only exist in one of the allowed states and not in any other state. History The old quantum theory was instigated by the 1900 work of Max Planck on the emission and absorption of light in a bla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Planck
Max Karl Ernst Ludwig Planck (, ; 23 April 1858 – 4 October 1947) was a German theoretical physicist whose discovery of energy quanta won him the Nobel Prize in Physics in 1918. Planck made many substantial contributions to theoretical physics, but his fame as a physicist rests primarily on his role as the originator of quantum theory, which revolutionized human understanding of atomic and subatomic processes. In 1948, the German scientific institution Kaiser Wilhelm Society (of which Planck was twice president) was renamed Max Planck Society (MPG). The MPG now includes 83 institutions representing a wide range of scientific directions. Life and career Planck came from a traditional, intellectual family. His paternal great-grandfather and grandfather were both theology professors in Göttingen; his father was a law professor at the University of Kiel and Munich. One of his uncles was also a judge. Planck was born in 1858 in Kiel, Holstein, to Johann Julius Wilhelm Pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arthur Erich Haas
Arthur Erich Haas (April 30, 1884 in Brno – February 20, 1941 in Chicago) was an Austrian physicist, noted for a 1910 paper he submitted in support of his habilitation as ''Privatdocent'' at the University of Vienna that outlined a treatment of the hydrogen atom involving quantization of electronic orbitals, thus anticipating the Bohr model (1913) by three years. Haas’ paper, however, was initially rejected and even ridiculed. As noted in his autobiography, Haas recalls: "When I lectured to the Chemical-Physical Society of Vienna ... Lecher ... referred to the presentation during open discussion as a carnival joke" (the lecture was held during carnival time in Austria, February 1910). Soon thereafter, however, by September 1911 at a physical science convention in Karlsruhe, former detractors of Haas' work acknowledged it with greater enthusiasm as noted in a footnote: "We do not know what caused change of mind in 1911 and can merely suggest the general trend of thinking a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an atom are a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence Shell
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can determine the element's chemical properties, such as its valence—whether it may bond with other elements and, if so, how readily and with how many. In this way, a given element's reactivity is highly dependent upon its electronic configuration. For a main-group element, a valence electron can exist only in the outermost electron shell; for a transition metal, a valence electron can also be in an inner shell. An atom with a closed shell of valence electrons (corresponding to a noble gas configuration) tends to be chemically inert. Atoms with one or two valence electrons more than a closed shell are highly reactive due to the rela ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Level
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized. In chemistry and atomic physics, an electron shell, or principal energy level, may be thought of as the orbit of one or more electrons around an atom's nucleus. The closest shell to the nucleus is called the " shell" (also called "K shell"), followed by the " shell" (or "L shell"), then the " shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond with the principal quan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, quantum field theory, quantum technology, and quantum information science. Classical physics, the collection of theories that existed before the advent of quantum mechanics, describes many aspects of nature at an ordinary (macroscopic) scale, but is not sufficient for describing them at small (atomic and subatomic) scales. Most theories in classical physics can be derived from quantum mechanics as an approximation valid at large (macroscopic) scale. Quantum mechanics differs from classical physics in that energy, momentum, angular momentum, and other quantities of a bound system are restricted to discrete values ( quantization); objects have characteristics of both particles and waves (wave–particle duality); and there are limits to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Obsolete Scientific Theory
This list catalogs well-accepted theories in science and pre-scientific natural philosophy and natural history which have since been superseded by scientific theories. Many discarded explanations were once supported by a scientific consensus, but replaced after more empirical information became available that identified flaws and prompted new theories which better explain the available data. Pre-modern explanations originated before the scientific method, with varying degrees of empirical support. Some theories are discarded in their entirety, such as the replacement of the phlogiston theory by energy and thermodynamics. Some theories known to be incomplete or in some ways incorrect are still used. For example, Newtonian classical mechanics is accurate enough for practical calculations at everyday distances and velocities, and it is still taught in schools. The more complicated relativistic mechanics must be used for long distances and velocities nearing the speed of light, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orders Of Approximation
In science, engineering, and other quantitative disciplines, order of approximation refers to formal or informal expressions for how accurate an approximation is. Usage in science and engineering In formal expressions, the ordinal number used before the word order refers to the highest power in the series expansion used in the approximation. The expressions: a ''zeroth-order approximation'', a ''first-order approximation'', a ''second-order approximation'', and so forth are used as fixed phrases. The expression a ''zero-order approximation'' is also common. Cardinal numerals are occasionally used in expressions like an ''order-zero approximation'', an ''order-one approximation'', etc. The omission of the word ''order'' leads to phrases that have less formal meaning. Phrases like first approximation or to a first approximation may refer to ''a roughly approximate value of a quantity''. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |