|
Barium Azide
Barium azide is an inorganic azide with the formula . It is a barium salt of hydrazoic acid. Like most azides, it is explosive. It is less sensitive to mechanical shock than lead azide. Preparation Barium azide may be prepared by reacting sodium azide with a soluble barium salt. Care should be taken to prevent large crystals from forming in the solution as barium azide crystals will explode if subjected to friction/shock or if fully dried. The product should be stored submerged in ethanol. Uses Barium azide can be used to make azides of magnesium, sodium, potassium, lithium, rubidium and zinc Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ... with their respective sulfates. : It can also be used as a source for high pure nitrogen by heating: : This reaction liberates metall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of UN Numbers 1601 To 1700
UN numbers from UN1601 to UN1700 as assigned by the United Nations Committee of Experts on the Transport of Dangerous Goods are as follows: __NOTOC__ UN 1601 to UN 1700 n.o.s. = ''not otherwise specified'' meaning a collective entry to which substances, mixtures, solutions or articles may be assigned if a) they are not mentioned by name in ''3.2 Dangerous Goods List'' AND b) they exhibit chemical, physical and/or dangerous properties corresponding to the Class, classification code, packing group and the name and description of the n.o.s. entry See also *Lists of UN numbers The UN numbers range from UN0001 to about UN3600 and are assigned by the United Nations Committee of Experts on the Transport of Dangerous Goods. UN 0001 to 0600 * List of UN numbers 0001 to 0100 * List of UN numbers 0101 to 0200 * List of UN nu ... References External linksADR Dangerous Goods cited on 11 May 2015.UN Dangerous Goods List from 2015 cited on 11 May 2015.UN Dangerous Goods List from 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Azide
Lithium azide is the lithium salt of hydrazoic acid. It is an unstable and toxic compound that decomposes into lithium and nitrogen when heated. Preparation It can be prepared by metathesis reaction between sodium azide and lithium nitrate or lithium sulfate solutions: : : It can also be prepared by reacting lithium sulfate with barium azide Barium azide is an inorganic azide with the formula . It is a barium salt of hydrazoic acid. Like most azides, it is explosive. It is less sensitive to mechanical shock than lead azide. Preparation Barium azide may be prepared by reacting sodi .... : References Lithium salts Azides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barium Compounds
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element. The most common minerals of barium are baryte ( barium sulfate, BaSO4) and witherite (barium carbonate, BaCO3). The name ''barium'' originates from the alchemical derivative "baryta", from Greek (), meaning 'heavy'. ''Baric'' is the adjectival form of barium. Barium was identified as a new element in 1774, but not reduced to a metal until 1808 with the advent of electrolysis. Barium has few industrial applications. Historically, it was used as a getter for vacuum tubes and in oxide form as the emissive coating on indirectly heated cathodes. It is a component of YBCO (high-temperature superconductors) and electroceramics, and is added to steel and cast iron to reduce the size of carbon grains within the microstructure. Barium compounds a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azides
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant application of azides is as a propellant in air bags. Preparation Sodium azide is made industrially by the reaction of nitrous oxide, with sodium amide in liquid ammonia as solvent: : Many inorganic azides can be prepared directly or indirectly from sodium azide. For example, lead azide, used in detonators, may be prepared from the metathesis reaction between lead nitrate and sodium azide. An alternative route is direct reaction of the metal with silver azide dissolved in liquid ammonia. Some azides are produced by treating the carbonate salts with hydrazoic acid. Bonding Azide is isoelectronic with carbon dioxide , cyanate , nitrous oxide , nitronium ion and cyanogen fluoride NCF. Per valence bond theory, azide can be described by sever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazoic Acid
Hydrazoic acid, also known as hydrogen azide or azoimide, This also contains a detailed description of the contemporaneous production process. is a compound with the chemical formula . It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes. Hydrazoic acid, like its fellow mineral acids, is soluble in water. Undiluted hydrazoic acid is dangerously explosive with a standard enthalpy of formation ΔfHo (l, 298K) = +264 kJ/mol. When dilute, the gas and aqueous solutions (<10%) can be safely prepared but should be used immediately; because of its low boiling point, hydrazoic acid is enriched upon evaporation and condensation such that dilute solutions incapable of explosion can form droplets in the headspace of the containe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
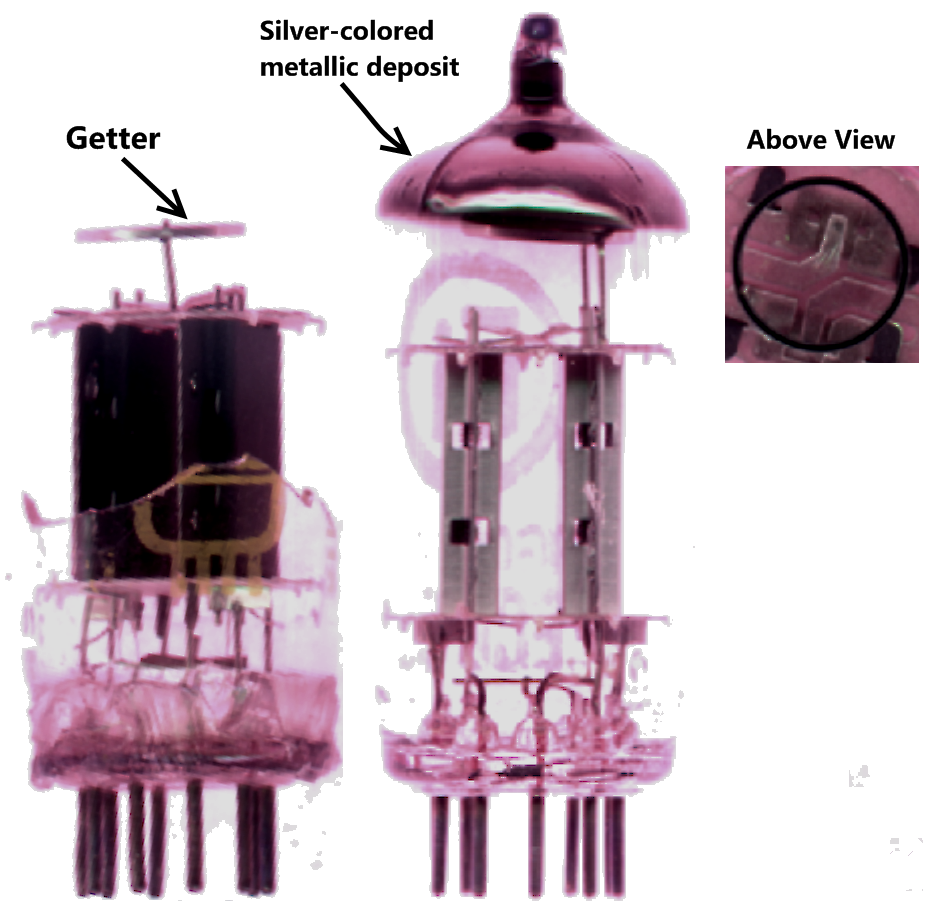
Getter
A getter is a deposit of reactive material that is placed inside a vacuum system to complete and maintain the vacuum. When gas molecules strike the getter material, they combine with it chemically or by . Thus the getter removes small amounts of gas from the evacuated space. The getter is usually a coating applied to a surface within the evacuated chamber. A vacuum is initially created by connecting a container to a vacuum pump. After achieving a sufficient vacuum, the container can be sealed, or the vacuum pump can be left running. Getters are especially important in sealed systems, such as vacuum tubes, including cathode ray tubes (CRTs), Vacuum Insulating Glass (or Vacuum Glass) and vacuum insulated panels, which must maintain a vacuum for a long time. This is because the inner surfaces of the container release adsorbed gases for a long time after the vacuum is established. The getter continually removes residues of a reactive gas, such as oxygen, as long as it is desorbed fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plenum Press
Springer Science+Business Media, commonly known as Springer, is a German multinational publishing company of books, e-books and peer-reviewed journals in science, humanities, technical and medical (STM) publishing. Originally founded in 1842 in Berlin, it expanded internationally in the 1960s, and through mergers in the 1990s and a sale to venture capitalists it fused with Wolters Kluwer and eventually became part of Springer Nature in 2015. Springer has major offices in Berlin, Heidelberg, Dordrecht, and New York City. History Julius Springer founded Springer-Verlag in Berlin in 1842 and his son Ferdinand Springer grew it from a small firm of 4 employees into Germany's then second largest academic publisher with 65 staff in 1872.Chronology ". Springer Science+Business Media. In 1964, Springer expanded its business international ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Azide
Zinc azide is an inorganic compound composed of zinc cations () and azide anions (). It is a white, explosive solid that can be prepared by the protonolysis of diethylzinc with hydrazoic acid: : Properties Zinc azide is a coordination polymer which crystallizes in three polymorphs, all of which feature tetrahedral zinc centers and bridging azide ligands. α- crystallizes in the monoclinic space group and is stable, while the other two polymorphs are metastable. ''P''21/''n''. β- is trigonal, space group ''P''3221, and γ- is monoclinic, space group ''C''2. It is easily hydrolyzed, and attempts to prepare it in aqueous solution resulted in the precipitation of basic azides (''x'' = 0.9–1.0). Both the α- and β-forms were found to be very friction- and shock-sensitive, violently exploding in blue flashes, but can be made to decompose slowly by gentle heating, giving off nitrogen gas. In a sealed glass tube with inert atmosphere, this yields zinc nitride, . Reference ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubidium Azide
Rubidium azide is an inorganic compound with the formula . It is the rubidium salt of the hydrazoic acid . Like most azides, it is explosive. Preparation Rubidium azide can be created by the reaction between rubidium sulfate and barium azide which results in formation of easily separated insoluble barium sulfate: : In at least one study, rubidium azide was produced by the reaction between butyl nitrite, hydrazine monohydrate, and rubidium hydroxide in the presence of ethanol: : This formula is typically used to synthesize potassium azide from caustic potash. Uses Rubidium azide has been investigated for possible use in alkali vapor cells, which are components of atomic clocks, atomic magnetometers and atomic gyroscopes. Azides are desirable starting materials because they decompose into rubidium metal and nitrogen gas when exposed to UV light. According to one publication:Among the different techniques used to fill microfabricated alkali vapor cell , UV decomposition of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Azide
Potassium azide is the inorganic compound having the formula . It is a white, water-soluble salt. It is used as a reagent in the laboratory. It has been found to act as a nitrification inhibitor in soil. Structure , , , and adopt the same structures. They crystallize in a tetragonal habit. The azide is bound to eight cations in an eclipsed orientation. The cations are bound to eight terminal N centers. Synthesis and reactions is prepared by treating potassium carbonate with hydrazoic acid, which is generated in situ. In contrast, the analogous sodium azide is prepared (industrially) by the " Wislicenus process," which proceeds via the reaction sodium amide with nitrous oxide.Horst H. Jobelius, Hans-Dieter Scharff "Hydrazoic Acid and Azides" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. Upon heating or upon irradiation with ultraviolet light, it decomposes into potassium metal and nitrogen gas. The decomposition temperatures of the alkali me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United States Army Research, Development And Engineering Command
The Combat Capabilities Development Command, (DEVCOM, aka CCDC) (formerly the United States Army Research, Development, and Engineering Command (RDECOM)) is a subordinate command of the U.S. Army Futures Command. RDECOM was tasked with "creating, integrating, and delivering technology-enabled solutions" to the U.S. Army. Headquartered at Aberdeen Proving Ground in Maryland, RDECOM employed more than 13,000 scientists, engineers, researchers, and support personnel working at six major RDE centers and at the U.S. Army Research Laboratory (ARL), providing nearly all of the Army's basic and applied research and development services, including in collaboration with other branches of the armed forces and through a network of more than a thousand academic, industrial, and international partners. CCDC now includes thDEVCOM Analysis Center(formerly ARL/SLAD and AMSAA), and aligns with the top six priorities of AFC: #Long Range Precision Fires (LRPF) #Next Generation Combat Vehicles (NGCV ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Azide
Magnesium azide is an inorganic chemical compound with the formula . It is composed of the magnesium cation () and the azide anions (). Properties Magnesium azide hydrolyzes easily. Like most azides, it is explosive. References azide magnesium Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ... Explosive chemicals {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |