|
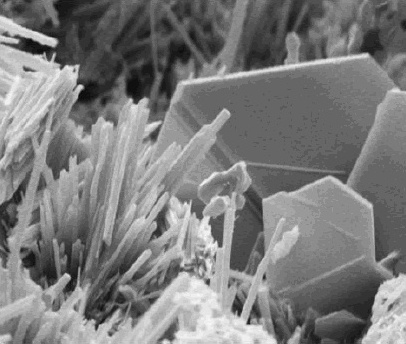
Baralyme
Baralyme is a mixture of 80% calcium hydroxide and 20% barium hydroxide compounds that is used as an alternative to soda lime Soda lime is a mixture of NaOH and CaO chemicals, used in granular form in closed breathing environments, such as general anaesthesia, submarines, rebreathers and recompression chambers, to remove carbon dioxide from breathing gases to prevent ... to absorb the exhaled carbon dioxide in a closed circuit anesthetic system.Mosby's Medical Dictionary, 8th edition. 2009, Elsevier. The substance has been used for carbon dioxide scrubbing in diving bells and the U.S Navy's engineered SEALAB's I, II, and the failed SEALAB III. References {{reflist Anesthesia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed or slaked with water. It has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide. Properties Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. With a solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the following dissolution reaction: : Ca(OH)2 → Ca2+ + 2 OH− At ambient temperature, calcium hydroxide (portlandite) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barium Hydroxide
Barium hydroxide is a chemical compound with the chemical formula Ba(OH)2. The monohydrate (''x'' = 1), known as baryta or baryta-water, is one of the principal compounds of barium. This white granular monohydrate is the usual commercial form. Preparation and structure Barium hydroxide can be prepared by dissolving barium oxide (BaO) in water: :BaO + H2O → Ba(OH)2 It crystallises as the octahydrate, which converts to the monohydrate upon heating in air. At 100 °C in a vacuum, the monohydrate will yield BaO and water. The monohydrate adopts a layered structure (see picture above). The Ba2+ centers adopt a square anti-prismatic geometry. Each Ba2+ center is bound by two water ligands and six hydroxide ligands, which are respectively doubly and triply bridging to neighboring Ba2+ centre sites. In the octahydrate, the individual Ba2+ centers are again eight coordinate but do not share ligands. Uses Industrially, barium hydroxide is used as the precursor to other bar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soda Lime
Soda lime is a mixture of NaOH and CaO chemicals, used in granular form in closed breathing environments, such as general anaesthesia, submarines, rebreathers and recompression chambers, to remove carbon dioxide from breathing gases to prevent CO2 retention and carbon dioxide poisoning. It is made by treating slaked lime with concentrated sodium hydroxide solution. Chemical components The main components of soda lime are * Calcium oxide, CaO (about 75%) * Water, H2O (about 20%) * Sodium hydroxide, NaOH (about 3%) * Potassium hydroxide, KOH (about 0.1%). Anaesthetic use During the administration of general anaesthesia, the gases expired by a patient, which contain carbon dioxide, are passed through an anaesthetic machine breathing circuit filled with soda lime granules. Medical-grade soda lime includes an indicating dye that changes color when the soda lime reaches its carbon dioxide absorbing capacity. To ensure that a soda lime canister (CO2 absorber) is functioning properly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide Scrubber
A carbon dioxide scrubber is a piece of equipment that absorbs carbon dioxide (CO2). It is used to treat exhaust gases from industrial plants or from exhaled air in life support systems such as rebreathers or in spacecraft, submersible craft or airtight chambers. Carbon dioxide scrubbers are also used in controlled atmosphere (CA) storage. They have also been researched for carbon capture and storage as a means of combating climate change. Technologies Amine scrubbing The primary application for CO2 scrubbing is for removal of CO2 from the exhaust of coal- and gas-fired power plants. Virtually the only technology being seriously evaluated involves the use of various amines, e.g. monoethanolamine. Cold solutions of these organic compounds bind CO2, but the binding is reversed at higher temperatures: :CO2 + 2 ↔ + , this technology has only been lightly implemented because of capital costs of installing the facility and the operating costs of utilizing it. Minerals and ze ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SEALAB
SEALAB I, II, and III were experimental underwater habitats developed by the United States Navy in the 1960s to prove the viability of saturation diving and humans living in isolation for extended periods of time. The knowledge gained from the SEALAB expeditions helped advance the science of deep sea diving and rescue, and contributed to the understanding of the psychological and physiological strains humans can endure. United States Navy Genesis Project Preliminary research work was undertaken by George F. Bond. Bond began investigations in 1957 to develop theories about saturation diving. Bond's team exposed rats, goats, monkeys, and human beings to various gas mixtures at different pressures. By 1963 they had collected enough data to test the first SEALAB habitat. SEALAB I SEALAB I was commanded by Captain Bond, who became known as "Papa Topside". SEALAB I proved that saturation diving in the open ocean was viable for extended periods. The experiment also offered info ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |