|
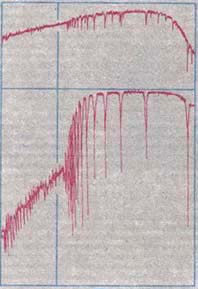
Balmer Discontinuity
The Balmer jump, Balmer discontinuity, or Balmer break is the difference of intensity of the stellar continuum spectrum on either side of the limit of the Balmer series of hydrogen, at approximately 364.5 nm. It is caused by electrons being completely ionized directly from the second energy level of a hydrogen atom (bound-free absorption), which creates a continuum absorption at wavelength In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats. It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, t ...s shorter than 364.5 nm. In some cases the Balmer discontinuity can show continuum emission (electromagnetic radiation), emission, usually when the Balmer lines themselves are strongly in emission. Other hydrogen spectral series also show bound-free absorption and hence a continuum discontinuity, but the Balmer jump in the near ultraviole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption (electromagnetic Radiation)
In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into internal energy of the absorber (for example, thermal energy). A notable effect is attenuation, or the gradual reduction of the intensity of light waves as they propagate through a medium. Although the absorption of waves does not usually depend on their intensity (linear absorption), in certain conditions (optics) the medium's transparency changes by a factor that varies as a function of wave intensity, and saturable absorption (or nonlinear absorption) occurs. Quantifying absorption Many approaches can potentially quantify radiation absorption, with key examples following. * The absorption coefficient along with some closely related derived quantities * The attenuation coefficient (NB used infrequently with meaning synonymous with "absorption coefficient") * The Molar attenuation coefficient (a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Luminosity
Luminosity is an absolute measure of radiated electromagnetic power (light), the radiant power emitted by a light-emitting object over time. In astronomy, luminosity is the total amount of electromagnetic energy emitted per unit of time by a star, galaxy, or other astronomical object. In SI units, luminosity is measured in joules per second, or watts. In astronomy, values for luminosity are often given in the terms of the luminosity of the Sun, ''L''⊙. Luminosity can also be given in terms of the astronomical magnitude system: the absolute bolometric magnitude (''M''bol) of an object is a logarithmic measure of its total energy emission rate, while absolute magnitude is a logarithmic measure of the luminosity within some specific wavelength range or filter band. In contrast, the term ''brightness'' in astronomy is generally used to refer to an object's apparent brightness: that is, how bright an object appears to an observer. Apparent brightness depends on both the lumin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Gravity
The surface gravity, ''g'', of an astronomical object is the gravitational acceleration experienced at its surface at the equator, including the effects of rotation. The surface gravity may be thought of as the acceleration due to gravity experienced by a hypothetical test particle which is very close to the object's surface and which, in order not to disturb the system, has negligible mass. For objects where the surface is deep in the atmosphere and the radius not known, the surface gravity is given at the 1 bar pressure level in the atmosphere. Surface gravity is measured in units of acceleration, which, in the SI system, are meters per second squared. It may also be expressed as a multiple of the Earth's standard surface gravity, which is equal to :''g'' = In astrophysics, the surface gravity may be expressed as log ''g'', which is obtained by first expressing the gravity in cgs units, where the unit of acceleration and surface gravity is centimeters per seco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematically, density is defined as mass divided by volume: : \rho = \frac where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate – this quantity is more specifically called specific weight. For a pure substance the density has the same numerical value as its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium and iridium are the densest known elements at standard conditions for temperature and pressure. To simplify comparisons of density across different s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer. Thermometers are calibrated in various temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), the latter being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible to extract energy as heat from a body at that temperature. Temperature is important in all fields of natur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nanometer, nm (with a corresponding frequency around 30 Hertz, PHz) to 400 nm (750 Hertz, THz), shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionization, ionize atoms, it can cause chemical reactions and causes many substances to glow or fluorescence, fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules. Short-wave ultraviolet light damages DNA and sterilizes surf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emission (electromagnetic Radiation)
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a transition from a high energy state to a lower energy state. The photon energy of the emitted photon is equal to the energy difference between the two states. There are many possible electron transitions for each atom, and each transition has a specific energy difference. This collection of different transitions, leading to different radiated wavelengths, make up an emission spectrum. Each element's emission spectrum is unique. Therefore, spectroscopy can be used to identify elements in matter of unknown composition. Similarly, the emission spectra of molecules can be used in chemical analysis of substances. Emission In physics, emission is the process by which a higher energy quantum mechanical state of a particle becomes converted to a lower one through the emission of a photon, resulting in the production of light. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats. It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, troughs, or zero crossings, and is a characteristic of both traveling waves and standing waves, as well as other spatial wave patterns. The inverse of the wavelength is called the spatial frequency. Wavelength is commonly designated by the Greek letter ''lambda'' (λ). The term ''wavelength'' is also sometimes applied to modulated waves, and to the sinusoidal envelopes of modulated waves or waves formed by interference of several sinusoids. Assuming a sinusoidal wave moving at a fixed wave speed, wavelength is inversely proportional to frequency of the wave: waves with higher frequencies have shorter wavelengths, and lower frequencies have longer wavelengths. Wavelength depends on the medium (for example, vacuum, air, or water) that a wav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Level
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized. In chemistry and atomic physics, an electron shell, or principal energy level, may be thought of as the orbit of one or more electrons around an atom's nucleus. The closest shell to the nucleus is called the " shell" (also called "K shell"), followed by the " shell" (or "L shell"), then the " shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond with the principal quan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiance
In radiometry, radiance is the radiant flux emitted, reflected, transmitted or received by a given surface, per unit solid angle per unit projected area. Radiance is used to characterize diffuse emission and reflection of electromagnetic radiation, and to quantify emission of neutrinos and other particles. The SI unit of radiance is the watt per steradian per square metre (). It is a ''directional'' quantity: the radiance of a surface depends on the direction from which it is being observed. The related quantity spectral radiance is the radiance of a surface per unit frequency or wavelength, depending on whether the spectrum is taken as a function of frequency or of wavelength. Historically, radiance was called "intensity" and spectral radiance was called "specific intensity". Many fields still use this nomenclature. It is especially dominant in heat transfer, astrophysics and astronomy. "Intensity" has many other meanings in physics, with the most common being power per unit ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionization
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected. Uses Everyday examples of gas ionization are such as within a fluorescent lamp or other electrical discharge lamps. It is also used in radiation detectors such as the Geiger-Müller counter or the ionization chamber. The ionizati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |