|
BRD3
Bromodomain-containing protein 3 (BRD3) also known as RING3-like protein (RING3L) is a protein that in humans is encoded by the BRD3 gene. This gene was identified based on its homology to the gene encoding the RING3 (BRD2) protein, a serine/threonine kinase. The gene maps to 9q34, a region which contains several major histocompatibility complex (MHC) genes. Structure BRD3 is a member of the Bromodomain and Extra-Terminal motif (BET) protein family. Like other BET family members it contains two tandem homologous bromodomains and an "Extra-Terminal" motif. BRD3, similar to BRD2, does not have a long C-terminal domain as BET family proteins BRD4 and BRDT do. Function Like other BET protein family members, BRD3 associates with acetylated lysine residues on histones and transcription factors. BRD3 has been implicated in nucleosome remodeling in the context of transcription. In addition, BRD3 has been shown to interact with RNA molecules and form protein-RNA aggregates. B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BRD2
Bromodomain-containing protein 2 is a protein that in humans is encoded by the ''BRD2'' gene. BRD2 is part of the Bromodomain and Extra-Terminal motif (BET) protein family that also contains BRD3, BRD4, and BRDT in mammals Early descriptions demonstrated that BRD2 gene product is a mitogen-activated kinase which localizes to the nucleus. The gene maps to the major histocompatibility complex (MHC) class II region on chromosome 6p21.3 but sequence comparison suggests that the protein is not involved in the immune response. Homology to the ''Drosophila'' gene female sterile homeotic suggests that this human gene may be part of a signal transduction pathway involved in growth control. Functions *BRD2 has been implicated in cancer. *BRD2 loss in mice causes obesity without diabetes for unknown reasons. *BRD2 may have functional overlap with close homolog BRD3. *BRD2 function is blocked by BET inhibitors. Interactions BRD2 has been shown to interact with E2F2, and many transcription ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BET Protein
A bromodomain is an approximately 110 amino acid protein domain that recognizes acetylated lysine residues, such as those on the ''N''-terminal tails of histones. Bromodomains, as the "readers" of lysine acetylation, are responsible in transducing the signal carried by acetylated lysine residues and translating it into various normal or abnormal phenotypes. Their affinity is higher for regions where multiple acetylation sites exist in proximity. This recognition is often a prerequisite for protein-histone association and chromatin remodeling. The domain itself adopts an all-α protein fold, a bundle of four alpha helices each separated by loop regions of variable lengths that form a hydrophobic pocket that recognizes the acetyl lysine. Discovery The bromodomain was identified as a novel structural motif by John W. Tamkun and colleagues studying the drosophila gene ''Brahma''/''brm'', and showed sequence similarity to genes involved in transcriptional activation. The name "bromod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromodomain
A bromodomain is an approximately 110 amino acid protein domain that recognizes acetylated lysine residues, such as those on the ''N''-terminal tails of histones. Bromodomains, as the "readers" of lysine acetylation, are responsible in transducing the signal carried by acetylated lysine residues and translating it into various normal or abnormal phenotypes. Their affinity is higher for regions where multiple acetylation sites exist in proximity. This recognition is often a prerequisite for protein-histone association and chromatin remodeling. The domain itself adopts an all-α protein fold, a bundle of four alpha helices each separated by loop regions of variable lengths that form a hydrophobic pocket that recognizes the acetyl lysine. Discovery The bromodomain was identified as a novel structural motif by John W. Tamkun and colleagues studying the drosophila gene ''Brahma''/''brm'', and showed sequence similarity to genes involved in transcriptional activation. The name "bromo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BRD4
Bromodomain-containing protein 4 is a protein that in humans is encoded by the ''BRD4'' gene. BRD4 is a member of the BET (bromodomain and extra terminal domain) family, which also includes BRD2, BRD3, and BRDT. BRD4, similar to other BET family members, contains two bromodomains that recognize acetylated lysine residues. BRD4 also has an extended C-terminal domain with little sequence homology to other BET family members. Structure The two bromodomains in BRD4, termed BD1 and BD2, consist of 4 alpha-helices linked by 2 loops. The ET domain structure is made up of 3 alpha-helices and a loop. The C-terminal domain of BRD4 has been implicated in promoting gene transcription through interaction with the transcription elongation factor P-TEFb and RNA polymerase II. Function The protein encoded by this gene is homologous to the murine protein MCAP, which associates with chromosomes during mitosis, and to the human BRD2 (RING3) protein, a serine/threonine kinase. Each of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BRDT
Bromodomain testis-specific protein is a protein that in humans is encoded by the ''BRDT'' gene. It is a member of the Bromodomain and Extra-terminal motif (BET) protein family. BRDT is similar to the RING3 protein family. It possesses 2 bromodomain motifs and a PEST sequence (a cluster of proline, glutamic acid, serine, and threonine residues), characteristic of proteins that undergo rapid intracellular degradation. The bromodomain is found in proteins that regulate transcription. Two transcript variants encoding the same protein have been found for this gene. The use of three different mouse models (Brdt knock-out mice, mice expressing a non-functional Brdt and mice expressing a mutated Brdt lacking its first bromodomain) showed that Brdt drives a meiotic and post-meiotic gene expression program. It also controls the genome-wide post-meiotic genome reorganization that occurs after histone hyperacetylation in elongating spermatids. Model organisms Model organisms have been us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NUT Midline Carcinoma
NUT carcinoma (NC; formerly NUT midline carcinoma (NMC)) is a rare genetically defined, very aggressive squamous cell epithelial cancer that usually arises in the midline of the body and is characterized by a chromosomal rearrangement in the nuclear protein in testis gene (i.e. ''NUTM1'' gene). In approximately 75% of cases, the coding sequence of ''NUTM1'' in band 14 on the long (or "q") arm of chromosome 15 is fused to '' BRD4'' or '' BRD3'', which creates a chimeric gene that encodes the ''BRD-NUT'' fusion protein. The remaining cases, the fusion of NUTM1 is to an unknown partner gene, usually called ''NUT''-variant. The name NUT carcinoma was introduced as the carcinoma does not only occur in the body midline; therefore, WHO also changed the name in 2015 in the WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Signs and symptoms In the United States, about 20–30 cases are reported each year. This may be a gross underestimate of the total number of cases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prostate Cancer
Prostate cancer is cancer of the prostate. Prostate cancer is the second most common cancerous tumor worldwide and is the fifth leading cause of cancer-related mortality among men. The prostate is a gland in the male reproductive system that surrounds the urethra just below the bladder. It is located in the hypogastric region of the abdomen. To give an idea of where it is located, the bladder is superior to the prostate gland as shown in the image The rectum is posterior in perspective to the prostate gland and the ischial tuberosity of the pelvic bone is inferior. Only those who have male reproductive organs are able to get prostate cancer. Most prostate cancers are slow growing. Cancerous cells may spread to other areas of the body, particularly the bones and lymph nodes. It may initially cause no symptoms. In later stages, symptoms include pain or difficulty urinating, blood in the urine, or pain in the pelvis or back. Benign prostatic hyperplasia may produce similar symptoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transcription Factor
In molecular biology, a transcription factor (TF) (or sequence-specific DNA-binding factor) is a protein that controls the rate of transcription of genetic information from DNA to messenger RNA, by binding to a specific DNA sequence. The function of TFs is to regulate—turn on and off—genes in order to make sure that they are expressed in the desired cells at the right time and in the right amount throughout the life of the cell and the organism. Groups of TFs function in a coordinated fashion to direct cell division, cell growth, and cell death throughout life; cell migration and organization (body plan) during embryonic development; and intermittently in response to signals from outside the cell, such as a hormone. There are up to 1600 TFs in the human genome. Transcription factors are members of the proteome as well as regulome. TFs work alone or with other proteins in a complex, by promoting (as an activator), or blocking (as a repressor) the recruitment of RNA ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
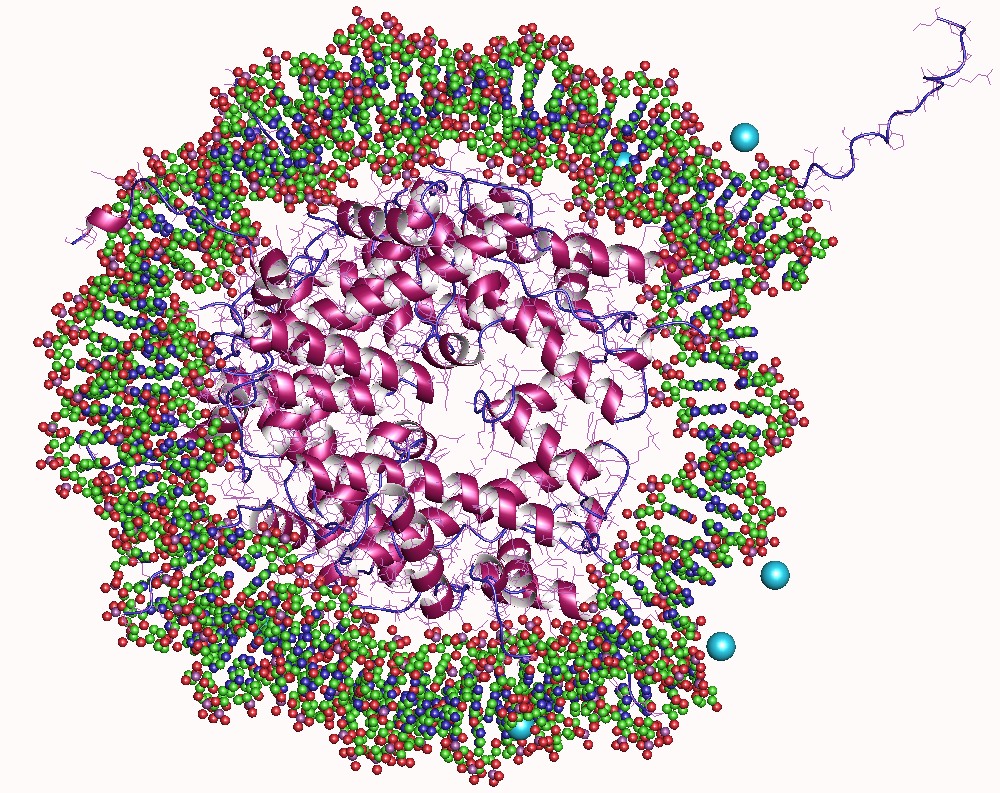
Histone
In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn are wrapped into 30-nanometer fibers that form tightly packed chromatin. Histones prevent DNA from becoming tangled and protect it from DNA damage. In addition, histones play important roles in gene regulation and DNA replication. Without histones, unwound DNA in chromosomes would be very long. For example, each human cell has about 1.8 meters of DNA if completely stretched out; however, when wound about histones, this length is reduced to about 90 micrometers (0.09 mm) of 30 nm diameter chromatin fibers. There are five families of histones which are designated H1/H5 (linker histones), H2, H3, and H4 (core histones). The nucleosome core is formed of two H2A-H2B dimers and a H3-H4 tetramer. The tight wrapping of DNA around histones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the ''S'' configuration. The human body cannot synthesize lysine. It is essential in humans and must therefore be obtained from the diet. In organisms that synthesise lysine, two main biosynthetic pathways exist, the diaminopimelate and α-aminoadipate pathways, which employ distinct e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylated
: In organic chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply ''acetates''. Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound. Organic synthesis Acetate esters and acetamides are generally prepared by acetylations. Acetylations are often used in making C-acetyl bonds in Friedel-Crafts reactions. Carbanions and their equivalents are susceptible to acetylations. Acetylation reagents Many acetylations are achieved using these three reagents: *Acetic anhydride. This reagent is common in the laboratory; its use cogenerates acetic acid. *Acetyl chloride. This reagent is also common in the laboratory, but its use cogenerates hydrogen chloride, which can be undesirable. *Ketene. At one time acetic anhydride was prepared by the reaction of ketene with acetic acid: :H2C=C=O + CH3COOH -> (CH3CO)2O\D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |