|
Bjerrum Length
The Bjerrum length (after Danish chemist Niels Bjerrum 1879–1958 ) is the separation at which the electrostatic interaction between two elementary charges is comparable in magnitude to the thermal energy scale, k_\text T, where k_\text is the Boltzmann constant and T is the absolute temperature in kelvins. This length scale arises naturally in discussions of electrostatic, electrodynamic and electrokinetic phenomena in electrolytes, polyelectrolytes and colloidal dispersions. In standard units, the Bjerrum length is given by \lambda_\text = \frac, where e is the elementary charge, \varepsilon_r is the relative dielectric constant of the medium and \varepsilon_0 is the vacuum permittivity. For water at room temperature \varepsilon_r \approx 80, so that In Gaussian units, 4\pi\varepsilon_0 = 1 and the Bjerrum length has the simpler form \lambda_\text = \frac. The relative permittivity ''ε''r of water decreases so strongly with temperature that the product (''ε''r· ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niels Bjerrum
Niels Janniksen Bjerrum (11 March 1879 in Copenhagen – 30 September 1958) was a Danish chemist. Niels Bjerrum was the son of ophthalmologist Jannik Petersen Bjerrum, and started to study at University of Copenhagen in 1897. He received his Master's degree in 1902 and his Doctor's degree in 1908, and did research in coordination complex chemistry under Sophus Mads Jørgensen. He became a docent in 1912, and in 1914 he became professor of chemistry at the Royal Agricultural College (''Landbohøjskolen'') in Copenhagen, as successor of Odin Tidemand Christensen. He stayed on this post until his retirement in 1949, and from 1939 to 1946 he was also the Director of the College. Importantly, Bjerrum introduced the concept of three forms of molecular energy, translational, vibrational and rotational which was important in understanding vibrational spectroscopy. He is also noted for the theory behind the Bjerrum length, and the Bjerrum plot. Bjerrum also performed some of the f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Permittivity
Vacuum permittivity, commonly denoted (pronounced "epsilon nought" or "epsilon zero"), is the value of the absolute dielectric permittivity of classical vacuum. It may also be referred to as the permittivity of free space, the electric constant, or the distributed capacitance of the vacuum. It is an ideal (baseline) physical constant. Its CODATA value is: : (farads per meter), with a relative uncertainty of It is a measure of how dense of an electric field is "permitted" to form in response to electric charges, and relates the units for electric charge to mechanical quantities such as length and force. For example, the force between two separated electric charges with spherical symmetry (in the vacuum of classical electromagnetism) is given by Coulomb's law: :F_\text = \frac \frac Here, ''q''1 and ''q''2 are the charges, ''r'' is the distance between their centres, and the value of the constant fraction 1/4 \pi \varepsilon_0 (known as the Coulomb constant, ''k''e) is ap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brownian Motion
Brownian motion, or pedesis (from grc, πήδησις "leaping"), is the random motion of particles suspended in a medium (a liquid or a gas). This pattern of motion typically consists of random fluctuations in a particle's position inside a fluid sub-domain, followed by a relocation to another sub-domain. Each relocation is followed by more fluctuations within the new closed volume. This pattern describes a fluid at thermal equilibrium, defined by a given temperature. Within such a fluid, there exists no preferential direction of flow (as in transport phenomena). More specifically, the fluid's overall linear and angular momenta remain null over time. The kinetic energies of the molecular Brownian motions, together with those of molecular rotations and vibrations, sum up to the caloric component of a fluid's internal energy (the equipartition theorem). This motion is named after the botanist Robert Brown, who first described the phenomenon in 1827, while looking t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
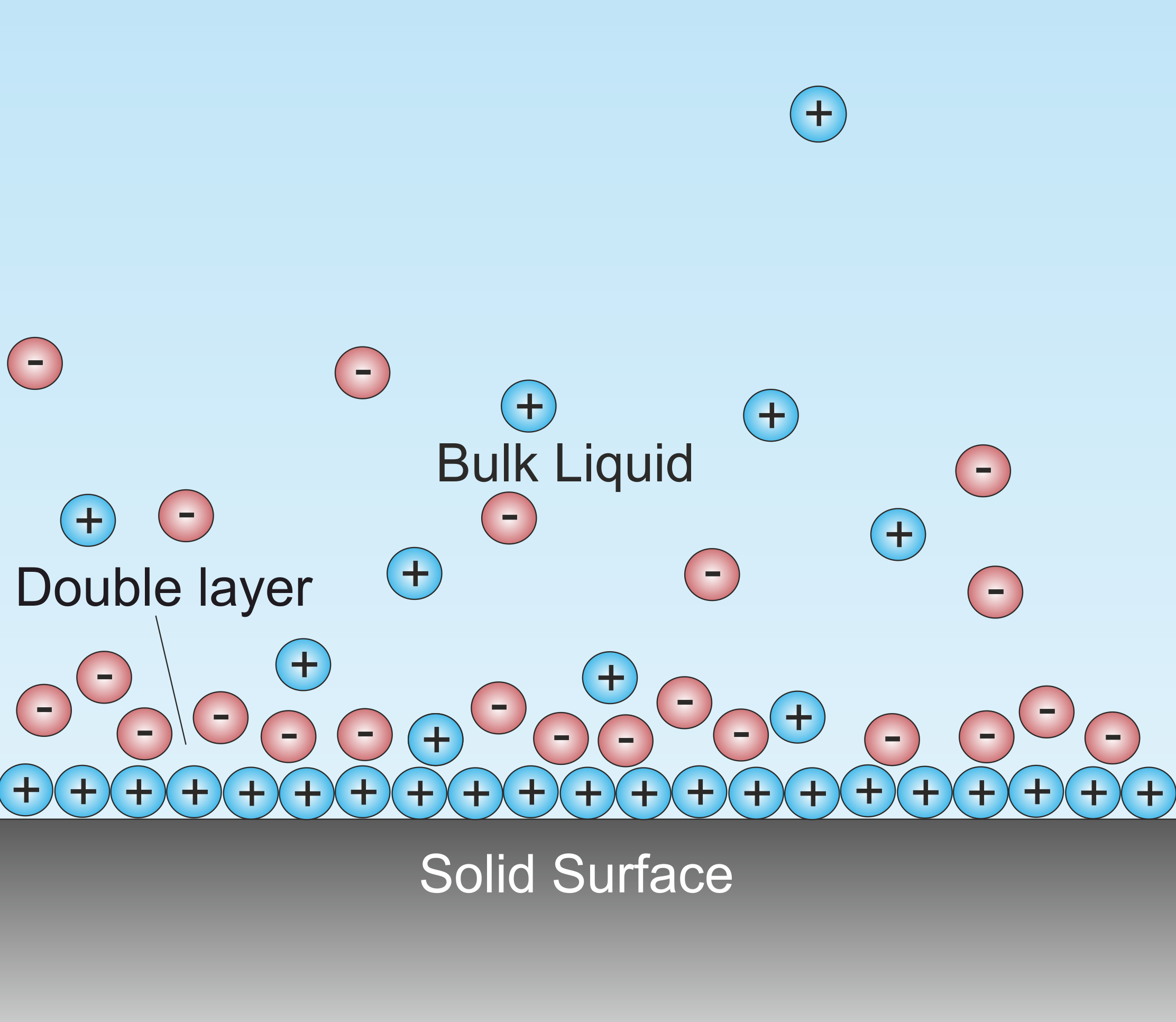
Double Layer (surface Science)
A double layer (DL, also called an electrical double layer, EDL) is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge (either positive or negative), consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer". Interfacial DLs are most apparent in systems with a large surface area to volume ratio, such as a colloid or porous bodies with particles or pores (respectively) on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric-field Screening
In physics, screening is the damping of electric fields caused by the presence of mobile charge carriers. It is an important part of the behavior of charge-carrying fluids, such as ionized gases (classical plasmas), electrolytes, and charge carriers in electronic conductors ( semiconductors, metals). In a fluid, with a given permittivity , composed of electrically charged constituent particles, each pair of particles (with charges and ) interact through the Coulomb force as \mathbf = \frac\hat, where the vector is the relative position between the charges. This interaction complicates the theoretical treatment of the fluid. For example, a naive quantum mechanical calculation of the ground-state energy density yields infinity, which is unreasonable. The difficulty lies in the fact that even though the Coulomb force diminishes with distance as , the average number of particles at each distance is proportional to , assuming the fluid is fairly isotropic. As a result, a charge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shielding Effect
In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an electron and the nucleus in any atom with more than one electron. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces on the electrons in the atom. It is a special case of electric-field screening. This effect also has some significance in many projects in material sciences. Strength per electron shell The wider the electron shells are in space, the weaker is the electric interaction between the electrons and the nucleus due to screening. In general we can order the electron shells (s,p,d,f) as such S(\mathrm) > S(\mathrm) > S(\mathrm) > S(\mathrm) , where ''S'' is the screening strength that a given orbital provides to the rest of the electrons. Description In hydrogen, or any other atom in group 1A of the periodic table (those with only one v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Debye–Hückel Equation
The chemists Peter Debye and Erich Hückel noticed that solutions that contain ionic solutes do not behave ideally even at very low concentrations. So, while the concentration of the solutes is fundamental to the calculation of the dynamics of a solution, they theorized that an extra factor that they termed gamma is necessary to the calculation of the activity coefficients of the solution. Hence they developed the Debye–Hückel equation and Debye–Hückel limiting law. The activity is only proportional to the concentration and is altered by a factor known as the activity coefficient \gamma. This factor takes into account the interaction energy of ions in solution. Debye–Hückel limiting law In order to calculate the activity a_C of an ion C in a solution, one must know the concentration and the activity coefficient: a_C = \gamma \frac\mathrm\mathrm, where * \gamma is the activity coefficient of C, * \mathrm is the concentration of the chosen ''standard state'', e.g. 1 mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Debye Length
In plasmas and electrolytes, the Debye length \lambda_ (also called Debye radius), is a measure of a charge carrier's net electrostatic effect in a solution and how far its electrostatic effect persists. With each Debye length the charges are increasingly electrically screened and the electric potential decreases in magnitude by 1/ e. A Debye sphere is a volume whose radius is the Debye length. Debye length is an important parameter in plasma physics, electrolytes, and colloids ( DLVO theory). The corresponding Debye screening wave vector k_=1/\lambda_ for particles of density n, charge q at a temperature T is given by k_^2=4\pi n q^2/(k_T) in Gaussian units. Expressions in MKS units will be given below. The analogous quantities at very low temperatures (T \to 0) are known as the Thomas–Fermi length and the Thomas–Fermi wave vector. They are of interest in describing the behaviour of electrons in metals at room temperature. The Debye length is named after the Dutch-Ame ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bjerrum Length In Water In Nanometers
{{disambig ...
Bjerrum may refer to: ;People * Niels Janniksen Bjerrum (1879–1958), Danish chemist (son of Jannik Petersen Bjerrum and father of Jannik Bjerrum) * Jannik Bjerrum (1909–1992), Danish chemist (son of Niels Janniksen Bjerrum) * Jannik Petersen Bjerrum (1851–1920), Danish ophthalmologist (father of Niels Janniksen Bjerrum) * Kirstine Bjerrum Meyer (1861–1941), Danish physicist (sister of Jannik Petersen Bjerrum) ;Other uses * Bjerrum plot * Bjerrum defect A Bjerrum defect is a crystallographic defect which is specific to ice, and which is partly responsible for the electrical properties of ice. It was first proposed by Niels Bjerrum in 1952 in order to explain the electrical polarization of ice in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gaussian Units
Gaussian units constitute a metric system of physical units. This system is the most common of the several electromagnetic unit systems based on cgs (centimetre–gram–second) units. It is also called the Gaussian unit system, Gaussian-cgs units, or often just cgs units. The term "cgs units" is ambiguous and therefore to be avoided if possible: there are several variants of cgs with conflicting definitions of electromagnetic quantities and units. SI units predominate in most fields, and continue to increase in popularity at the expense of Gaussian units. Alternative unit systems also exist. Conversions between quantities in Gaussian and SI units are direct unit conversions, because the quantities themselves are defined differently in each system. This means that the equations expressing physical laws of electromagnetism—such as Maxwell's—will change depending on the system of units employed. As an example, quantities that are dimensionless in one system may have dimension ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dielectric Constant
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric constant of an insulator measures the ability of the insulator to store electric energy in an electrical field. Permittivity is a material's property that affects the Coulomb force between two point charges in the material. Relative permittivity is the factor by which the electric field between the charges is decreased relative to vacuum. Likewise, relative permittivity is the ratio of the capacitance of a capacitor using that material as a dielectric, compared with a similar capacitor that has vacuum as its dielectric. Relative permittivity is also commonly known as the dielectric constant, a term still used but deprecated by standards organizations in engineering as well as in chemistry. Definition Relative permittivity is typically denoted as (sometimes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrostatic
Electrostatics is a branch of physics that studies electric charges at rest ( static electricity). Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word for amber, (), was thus the source of the word 'electricity'. Electrostatic phenomena arise from the forces that electric charges exert on each other. Such forces are described by Coulomb's law. Even though electrostatically induced forces seem to be rather weak, some electrostatic forces are relatively large. The force between an electron and a proton, which together make up a hydrogen atom, is about 36 orders of magnitude stronger than the gravitational force acting between them. There are many examples of electrostatic phenomena, from those as simple as the attraction of plastic wrap to one's hand after it is removed from a package, to the apparently spontaneous explosion of grain silos, the damage of electronic components during manufa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |