|
Biochemistry Of Alzheimer's Disease
The biochemistry of Alzheimer's disease, the most common cause of dementia, is not yet very well understood. Alzheimer's disease (AD) has been identified as a proteopathy: a protein misfolding disease due to the accumulation of abnormally folded amyloid beta (Aβ) protein in the brain. Amyloid beta is a short peptide that is an abnormal proteolytic byproduct of the transmembrane protein amyloid-beta precursor protein (APP), whose function is unclear but thought to be involved in neuronal development. The presenilins are components of proteolytic complex involved in APP processing and degradation. Amyloid beta monomers are soluble and contain short regions of beta sheet and polyproline II helix secondary structures in solution, though they are largely alpha helical in membranes; however, at sufficiently high concentration, they undergo a dramatic conformational change to form a beta sheet-rich tertiary structure that aggregates to form amyloid fibrils. These fibrils and o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dementia
Dementia is a disorder which manifests as a set of related symptoms, which usually surfaces when the brain is damaged by injury or disease. The symptoms involve progressive impairments in memory, thinking, and behavior, which negatively affects a person's ability to function and carry out everyday activities. Aside from memory impairment and a disruption in thought patterns, the most common symptoms include emotional problems, difficulties with language, and decreased motivation. The symptoms may be described as occurring in a continuum over several stages. Consciousness is not affected. Dementia ultimately has a significant effect on the individual, caregivers, and on social relationships in general. A diagnosis of dementia requires the observation of a change from a person's usual mental functioning, and a greater cognitive decline than what is caused by normal aging. Several diseases and injuries to the brain, such as a stroke, can give rise to dementia. However, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary Structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may interact and bond in a number of ways. The interactions and bonds of side chains within a particular protein determine its tertiary structure. The protein tertiary structure is defined by its atomic coordinates. These coordinates may refer either to a protein domain or to the entire tertiary structure.Branden C. and Tooze J. "Introduction to Protein Structure" Garland Publishing, New York. 1990 and 1991. A number of tertiary structures may fold into a quaternary structure.Kyte, J. "Structure in Protein Chemistry." Garland Publishing, New York. 1995. History The science of the tertiary structure of proteins has progressed from one of hypothesis to one of detailed definition. Although Emil Fischer had suggested proteins were made of poly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
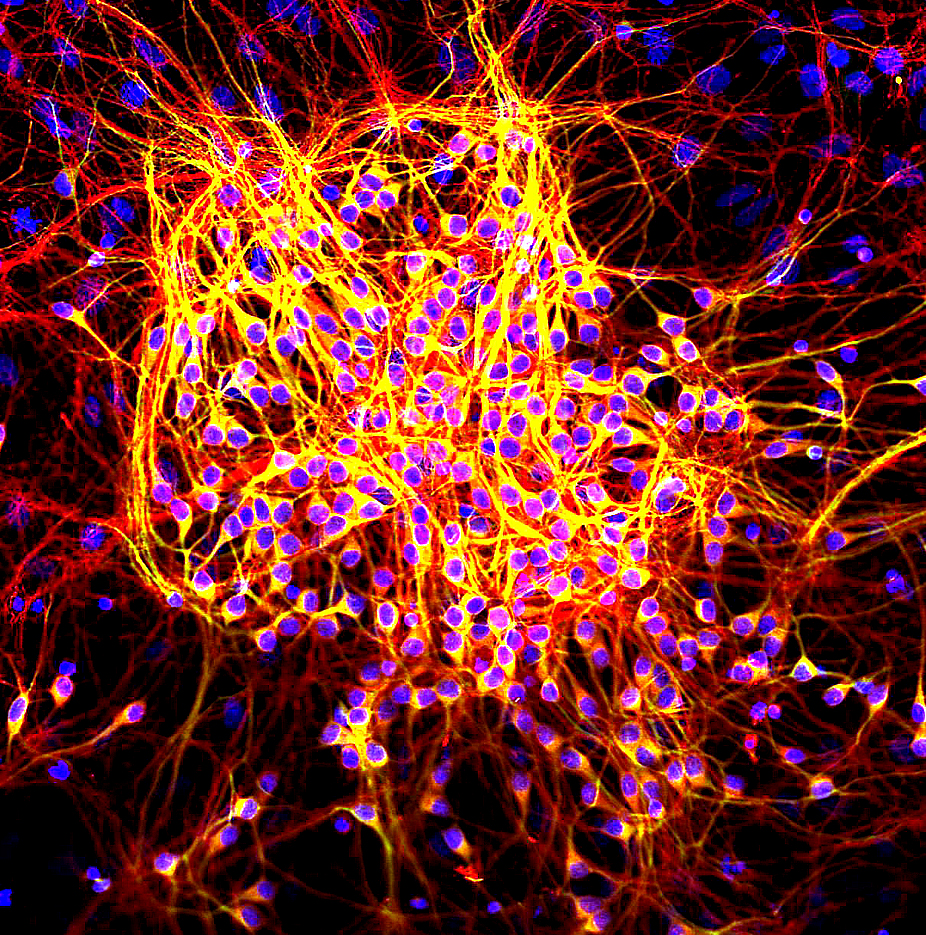
Neurite
A neurite or neuronal process refers to any projection from the cell body of a neuron. This projection can be either an axon or a dendrite. The term is frequently used when speaking of immature or developing neurons, especially of cells in culture, because it can be difficult to tell axons from dendrites before differentiation is complete. Neurite development The development of a neurite requires a complex interplay of both extracellular and intracellular signals. At every given point along a developing neurite, there are receptors detecting both positive and negative growth cues from every direction in the surrounding space. The developing neurite sums together all of these growth signals in order to determine which direction the neurite will ultimately grow towards. While not all of the growth signals are known, several have been identified and characterized. Among the known extracellular growth signals are netrin, a midline chemoattractant, and semaphorin, ephrin and col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurofibrillary Tangles
Neurofibrillary tangles (NFTs) are aggregates of hyperphosphorylated tau protein that are most commonly known as a primary biomarker of Alzheimer's disease. Their presence is also found in numerous other diseases known as tauopathies. Little is known about their exact relationship to the different pathologies. Formation Neurofibrillary tangles are formed by hyperphosphorylation of a microtubule-associated protein known as tau, causing it to aggregate, or group, in an insoluble form. (These aggregations of hyperphosphorylated tau protein are also referred to as PHF, or "paired helical filaments"). The precise mechanism of tangle formation is not completely understood, and it is still controversial whether tangles are a primary causative factor in disease or play a more peripheral role. Cytoskeletal changes Three different maturation states of NFT have been defined using anti-tau and anti-ubiquitin immunostaining. At stage 0 there are morphologically normal pyramidal cells sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. Protein phosphorylation often activates (or deactivates) many enzymes. Glucose Phosphorylation of sugars is often the first stage in their catabolism. Phosphorylation allows cells to accumulate sugars because the phosphate group prevents the molecules from diffusing back across their transporter. Phosphorylation of glucose is a key reaction in sugar metabolism. The chemical equation for the conversion of D-glucose to D-glucose-6-phosphate in the first step of glycolysis is given by :D-glucose + ATP → D-glucose-6-phosphate + ADP :ΔG° = −16.7 kJ/mol (° indicates measurement at standard condition) Hepatic cells are freely permeable to glucose, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoskeleton
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components, microfilaments, intermediate filaments and microtubules, and these are all capable of rapid growth or disassembly dependent on the cell's requirements. A multitude of functions can be performed by the cytoskeleton. Its primary function is to give the cell its shape and mechanical resistance to deformation, and through association with extracellular connective tissue and other cells it stabilizes entire tissues. The cytoskeleton can also contract, thereby deforming the cell and the cell's environment and allowing cells to migrate. Moreover, it is involved in many cell signaling pathways and in the uptake of extracellular material (endocytosis), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microtubules
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement. Microtubules play an important role in a number of cellular processes. They are involved in maintaining the structure of the cell and, together with microfilaments and intermediate filaments, they form the cytoskeleton. They also make up the internal structure of cilia and flagella. They provide platforms for intracellular transport and are involved in a variety of cellular processes, including the movement of secretory vesicles, or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microtubule-associated Protein
In cell biology, microtubule-associated proteins (MAPs) are proteins that interact with the microtubules of the cellular cytoskeleton. MAPs are integral to: the stability of the cell and its internal structures and the transport of components within the cell Function MAPs bind to the tubulin subunits that make up microtubules to regulate their stability. A large variety of MAPs have been identified in many different cell types, and they have been found to carry out a wide range of functions. These include both stabilizing and destabilizing microtubules, guiding microtubules towards specific cellular locations, cross-linking microtubules and mediating the interactions of microtubules with other proteins in the cell. Within the cell, MAPs bind directly to the tubulin dimers of microtubules. This binding can occur with either polymerized or depolymerized tubulin, and in most cases leads to the stabilization of microtubule structure, further encouraging polymerization. Usually, it is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tau Protein
The tau proteins (abbreviated from tubulin associated unit) are a group of six highly soluble protein isoforms produced by alternative splicing from the gene ''MAPT'' (microtubule-associated protein tau). They have roles primarily in maintaining the stability of microtubules in axons and are abundant in the neurons of the central nervous system (CNS), where the cerebral cortex has the highest abundance. They are less common elsewhere but are also expressed at very low levels in CNS astrocytes and oligodendrocytes. Pathologies and dementias of the nervous system such as Alzheimer's disease and Parkinson's disease are associated with tau proteins that have become hyperphosphorylated insoluble aggregates called neurofibrillary tangles. The tau proteins were identified in 1975 as heat-stable proteins essential for microtubule assembly, and since then they have been characterized as intrinsically disordered proteins. Function Microtubule stabilization Tau proteins are found mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tauopathy
Tauopathy belongs to a class of neurodegenerative diseases involving the aggregation of tau protein into neurofibrillary or gliofibrillary tangles in the human brain. Tangles are formed by hyperphosphorylation of the microtubule protein known as tau, causing the protein to dissociate from microtubules and form insoluble aggregates. (These aggregations are also called paired helical filaments.) The mechanism of tangle formation is not well understood, and whether tangles are a primary cause of Alzheimer's disease or play a peripheral role is unknown. Detection and imaging ;Post-mortem: Tau tangles are seen microscopically in stained brain samples. ;Pre-mortem: In living patients tau tangle locations can be imaged with a PET scan using a suitable radio-emissive agent. Alzheimer's disease Neurofibrillary tangles were first described by Alois Alzheimer in one of his patients with Alzheimer's disease (AD). The tangles are considered a secondary tauopathy. AD is also classi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerebral Amyloid Angiopathy
Cerebral amyloid angiopathy (CAA) is a form of angiopathy in which amyloid beta peptide deposits in the walls of small to medium blood vessels of the central nervous system and meninges. The term ''congophilic'' is sometimes used because the presence of the abnormal aggregations of amyloid can be demonstrated by microscopic examination of brain tissue after staining with Congo red. The amyloid material is only found in the brain and as such the disease is not related to other forms of amyloidosis. Signs and symptoms CAA is associated with brain hemorrhages, particularly microhemorrhages. Since CAA can be caused by the same amyloid protein that is associated with Alzheimer's dementia, brain bleeds are more common in people who have a diagnosis of Alzheimer's disease. However, they can also occur in those who have no history of dementia. The bleeding within the brain is usually confined to a particular lobe and this is slightly different compared to brain bleeds which occur as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neuritic Plaque
Amyloid plaques (also known as neuritic plaques, amyloid beta plaques or senile plaques) are extracellular deposits of the amyloid beta (Aβ) protein mainly in the grey matter of the brain. Degenerative neuronal elements and an abundance of microglia and astrocytes can be associated with amyloid plaques. Some plaques occur in the brain as a result of aging, but large numbers of plaques and neurofibrillary tangles are characteristic features of Alzheimer's disease. Abnormal neurites in amyloid plaques are tortuous, often swollen axons and dendrites. The neurites contain a variety of organelles and cellular debris, and many of them include characteristic paired helical filaments, the ultrastructural component of neurofibrillary tangles. The plaques are highly variable in shape and size; in tissue sections immunostained for Aβ, they comprise a log-normal size distribution curve with an average plaque area of 400-450 square micrometers (µm²). The smallest plaques (les ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |