|
Asperlicin
Asperlicin is a mycotoxin, derived from the fungus '' Aspergillus alliaceus''. It acts as a selective antagonist for the cholecystokinin receptor CCKA, and has been used as a lead compound A lead compound (, i.e. a "leading" compound, not to be confused with various compounds of the metallic element lead) in drug discovery is a chemical compound that has pharmacology, pharmacological or biological activity likely to be therapeutical ... for the development of a number of novel CCKA antagonists with potential clinical applications.Lattmann E, Billington DC, Poyner DR, Howitt SB, Offel M. Synthesis and evaluation of asperlicin analogues as non-peptidal cholecystokinin-antagonists. ''Drug Design and Discovery''. 2001;17(3):219-30. He et al. 1998 present a synthesis from aryl iodide and vinyl iodide. References Mycotoxins Cholecystokinin antagonists Lactams {{gastrointestinal-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholecystokinin A Receptor
The Cholecystokinin A receptor is a human protein, also known as CCKAR or CCK1, with CCK1 now being the IUPHAR-recommended name. Function This gene encodes a G-protein coupled receptor that binds sulfated members of the cholecystokinin (CCK) family of peptide hormones. This receptor is a major physiologic mediator of pancreatic enzyme secretion and smooth muscle contraction of the gallbladder and stomach. In the central and peripheral nervous system this receptor regulates satiety and the release of beta-endorphin and dopamine. The extracellular, N-terminal, domain of this protein adopts a tertiary structure consisting of a few helical turns and a disulfide-cross linked loop. It is required for interaction of the cholecystokinin A receptor with its corresponding hormonal ligand. Selective Ligands Agonists * Cholecystokinin Cholecystokinin (CCK or CCK-PZ; from Greek ''chole'', "bile"; ''cysto'', "sac"; ''kinin'', "move"; hence, ''move the bile-sac (gallbladder)'') is a pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycotoxin
A mycotoxin (from the Greek μύκης , "fungus" and τοξίνη , "toxin") is a toxic secondary metabolite produced by organisms of kingdom Fungi and is capable of causing disease and death in both humans and other animals. The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops. Examples of mycotoxins causing human and animal illness include aflatoxin, citrinin, fumonisins, ochratoxin A, patulin, trichothecenes, zearalenone, and ergot alkaloids such as ergotamine. One mold species may produce many different mycotoxins, and several species may produce the same mycotoxin. Production Most fungi are aerobic (use oxygen) and are found almost everywhere in extremely small quantities due to the diminute size of their spores. They consume organic matter wherever humidity and temperature are sufficient. Where conditions are right, fungi proliferate into colonies and mycotoxin levels become high. The reason for the product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspergillus Alliaceus
''Aspergillus alliaceus'' is a species of fungus in the genus ''Aspergillus''. It is from the ''Flavi'' section. It was first described scientifically by Charles Thom and Margaret Church in 1926. Its associated teleomorph In mycology, the terms teleomorph, anamorph, and holomorph apply to portions of the life cycles of fungi in the phyla Ascomycota and Basidiomycota: *Teleomorph: the sexual reproductive stage (morph), typically a fruiting body. *Anamorph: an ase ... is ''Petromyces alliaceus''. It has yellow spores. Growth and morphology ''A. alliaceus'' has been cultivated on both Czapek yeast extract agar (CYA) plates and Malt Extract Agar Oxoid® (MEAOX) plates. The growth morphology of the colonies can be seen in the pictures below. Aspergillus_alliaceus_cya.png, ''Aspergillus alliaceus'' growing on CYA plate Aspergillus_alliaceus_meaox.png, ''Aspergillus alliaceus'' growing on MEAOX plate References alliaceus Fungi described in 1926 Taxa named by Charles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholecystokinin Receptor
Cholecystokinin receptors or CCK receptors are a group of G-protein coupled receptors which bind the peptide hormones cholecystokinin (CCK) and gastrin. There are two different subtypes CCKA and CCKB which are ~50% homologous: Various cholecystokinin antagonists have been developed and are used in research, although the only drug of this class that has been widely marketed to date is the anti-ulcer drug proglumide Proglumide (Milid) is a drug that inhibits gastrointestinal motility and reduces gastric secretions. It acts as a cholecystokinin antagonist, which blocks both the CCKA and CCKB subtypes. It was used mainly in the treatment of stomach ulcers, .... References External links * G protein-coupled receptors {{Cell-biology-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead Compound
A lead compound (, i.e. a "leading" compound, not to be confused with various compounds of the metallic element lead) in drug discovery is a chemical compound that has pharmacological or biological activity likely to be therapeutically useful, but may nevertheless have suboptimal structure that requires modification to fit better to the target; lead drugs offer the prospect of being followed by back-up compounds. Its chemical structure serves as a starting point for chemical modifications in order to improve potency, selectivity, or pharmacokinetic parameters. Furthermore, newly invented pharmacologically active moieties may have poor druglikeness and may require chemical modification to become drug-like enough to be tested biologically or clinically. Terminology Lead compounds are sometimes called developmental candidates. This is because the discovery and selection of lead compounds occurs prior to preclinical and clinical development of the candidate. Discovering lead compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Modelling
Molecular modelling encompasses all methods, theoretical and computational, used to model or mimic the behaviour of molecules. The methods are used in the fields of computational chemistry, drug design, computational biology and materials science to study molecular systems ranging from small chemical systems to large biological molecules and material assemblies. The simplest calculations can be performed by hand, but inevitably computers are required to perform molecular modelling of any reasonably sized system. The common feature of molecular modelling methods is the atomistic level description of the molecular systems. This may include treating atoms as the smallest individual unit (a molecular mechanics approach), or explicitly modelling protons and neutrons with its quarks, anti-quarks and gluons and electrons with its photons (a quantum chemistry approach). Molecular mechanics Molecular mechanics is one aspect of molecular modelling, as it involves the use of classical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl Iodide
In organic chemistry, an aryl halide (also known as haloarene) is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications. Preparation The two main preparatory routes to aryl halides are direct halogenation and via diazonium salts. Direct halogenation In the Friedel-Crafts halogenation, Lewis acids serve as catalysts. Many metal chlorides are used, examples include iron(III) chloride or aluminium chloride. The most important aryl halide, chlorobenzene is produced by this route. Monochlorination of benzene is always accompanied by formation of the dichlorobenzene derivatives. Arenes with electron donating groups react with halogens even in the absence of Lew ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
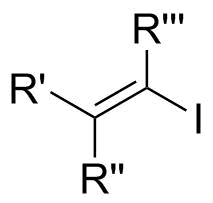
Vinyl Iodide
In organic chemistry, a vinyl iodide (also known as an iodoalkene) functional group is an alkene with one or more iodide substituents. Vinyl iodides are versatile molecules that serve as important building blocks and precursors in organic synthesis. They are commonly used in carbon-carbon forming reactions in transition-metal catalyzed cross-coupling reactions, such as Stille reaction, Heck reaction, Sonogashira coupling, and Suzuki coupling. Synthesis of well-defined geometry or complexity vinyl iodide is important in stereoselective synthesis of natural products and drugs. Properties Vinyl iodides are generally stable under nucleophilic conditions. In SN2 reactions, back-attack is difficult because of steric clash of R groups on carbon adjacent to electrophilic center (see figure 1a).Klein, David. Organic Chemistry. John Wiley & Sons, Jun 15, 2011. Google book. Thurs. 28 Nov. 2013. https://books.google.com/books?id=SsX9pbarkQkC&source=gbs_navlinks_s In addition, the lone pair ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reviews
''Chemical Reviews'' is peer-reviewed scientific journal published twice per month by the American Chemical Society. It publishes review articles on all aspects of chemistry. It was established in 1924 by William Albert Noyes (University of Illinois). the editor-in-chief is Sharon Hammes-Schiffer. Abstracting and indexing The journal is abstracted and indexed in Chemical Abstracts Service, CAB International, EBSCOhost, ProQuest, PubMed, Scopus, and the Science Citation Index. According to the ''Journal Citation Reports'', the journal has a 2020 impact factor of 60.622. See also * Accounts of Chemical Research ''Accounts of Chemical Research'' is a semi-monthly peer-reviewed scientific journal published by the American Chemical Society containing overviews of basic research and applications in chemistry and biochemistry. It was established in 1968 and th ... References External links * American Chemical Society academic journals Review journals Monthly journals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
American Chemical Society
The American Chemical Society (ACS) is a scientific society based in the United States that supports scientific inquiry in the field of chemistry. Founded in 1876 at New York University, the ACS currently has more than 155,000 members at all degree levels and in all fields of chemistry, chemical engineering, and related fields. It is one of the world's largest scientific societies by membership. The ACS is a 501(c) organization, 501(c)(3) non-profit organization and holds a congressional charter under Title 36 of the United States Code. Its headquarters are located in Washington, D.C., and it has a large concentration of staff in Columbus, Ohio. The ACS is a leading source of scientific information through its peer-reviewed scientific journals, national conferences, and the Chemical Abstracts Service. Its publications division produces over 60 Scientific journal, scholarly journals including the prestigious ''Journal of the American Chemical Society'', as well as the weekly tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycotoxins
A mycotoxin (from the Greek μύκης , "fungus" and τοξίνη , "toxin") is a toxic secondary metabolite produced by organisms of kingdom Fungi and is capable of causing disease and death in both humans and other animals. The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops. Examples of mycotoxins causing human and animal illness include aflatoxin, citrinin, fumonisins, ochratoxin A, patulin, trichothecenes, zearalenone, and ergot alkaloids such as ergotamine. One mold species may produce many different mycotoxins, and several species may produce the same mycotoxin. Production Most fungi are aerobic (use oxygen) and are found almost everywhere in extremely small quantities due to the diminute size of their spores. They consume organic matter wherever humidity and temperature are sufficient. Where conditions are right, fungi proliferate into colonies and mycotoxin levels become high. The reason for the product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholecystokinin Antagonists
Cholecystokinin (CCK or CCK-PZ; from Greek ''chole'', "bile"; ''cysto'', "sac"; ''kinin'', "move"; hence, ''move the bile-sac (gallbladder)'') is a peptide hormone of the gastrointestinal system responsible for stimulating the digestion of fat and protein. Cholecystokinin, formerly called pancreozymin, is synthesized and secreted by enteroendocrine cells in the duodenum, the first segment of the small intestine. Its presence causes the release of digestive enzymes and bile from the pancreas and gallbladder, respectively, and also acts as a hunger suppressant. History Evidence that the small intestine controls the release of bile was uncovered as early as 1856, when French physiologist Claude Bernard showed that when dilute acetic acid was applied to the orifice of the bile duct, the duct released bile into the duodenum. In 1903 the French physiologist showed that this reflex was not mediated by the nervous system. In 1904 the French physiologist Charles Fleig showed that the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |