|
Aplenzin
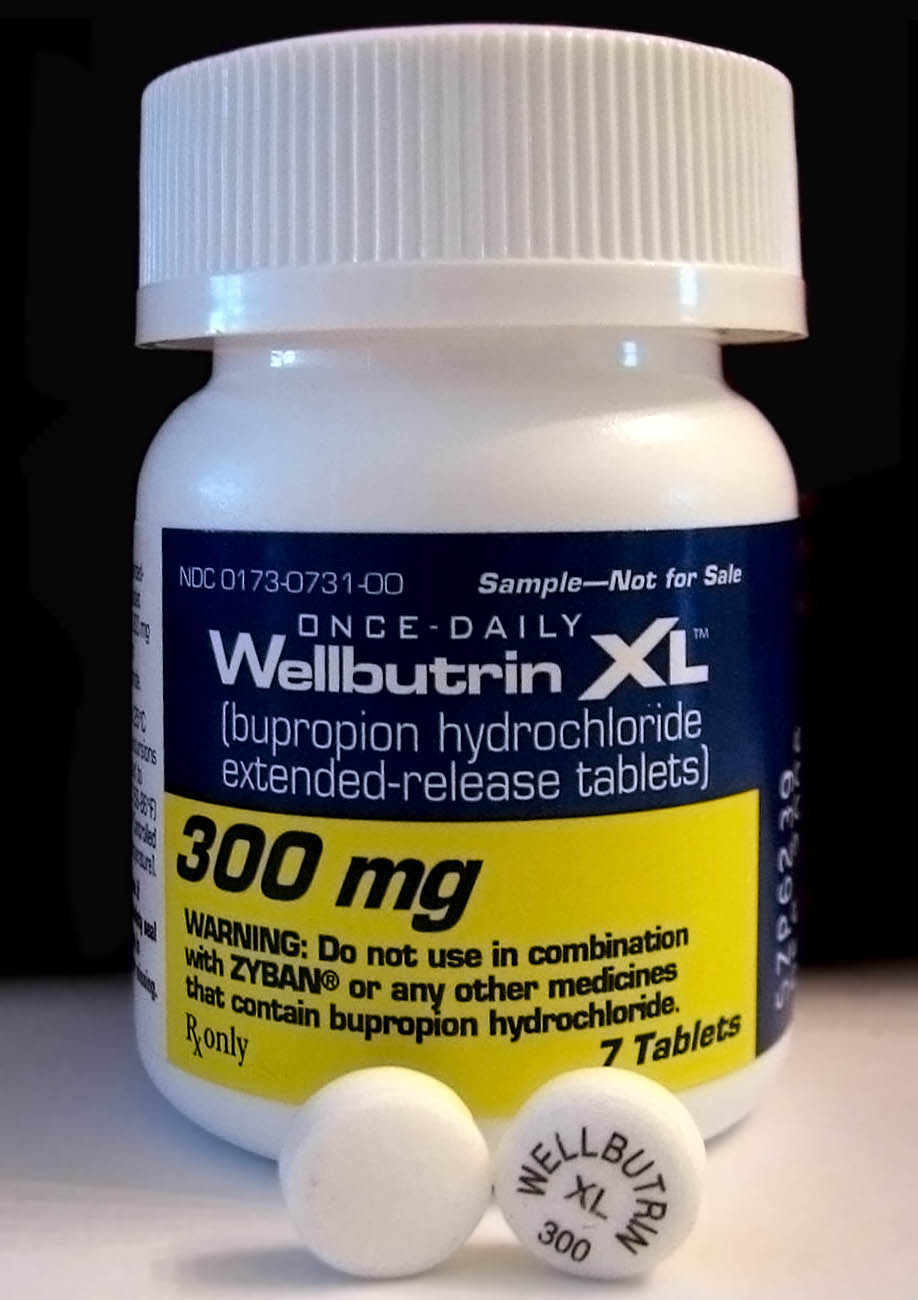
Bupropion, sold under the brand names Wellbutrin and Zyban among others, is an atypical antidepressant primarily used to treat major depressive disorder and to support smoking cessation. It is also popular as an add-on medication in the cases of "incomplete response" to the first-line selective serotonin reuptake inhibitor (SSRI) antidepressant. Bupropion has several features that distinguish it from other antidepressants: it does not usually cause sexual dysfunction; it is not associated with weight gain and sleepiness, and it is more effective than SSRIs at improving symptoms of hypersomnia and fatigue. Bupropion does, however, carry a much higher risk of seizure than many other antidepressants and extreme caution must be taken in patients with a history of seizure disorder. Common adverse effects of bupropion with the greatest difference from placebo are dry mouth, nausea, constipation, insomnia, anxiety, tremor, and excessive sweating. Raised blood pressure is notable. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepatotoxicity
Hepatotoxicity (from ''hepatic toxicity'') implies chemical-driven liver damage. Drug-induced liver injury is a cause of acute and chronic liver disease caused specifically by medications and the most common reason for a drug to be withdrawn from the market after approval. The liver plays a central role in transforming and clearing chemicals and is susceptible to the toxicity from these agents. Certain medicinal agents, when taken in overdoses (e.g. paracetamol) and sometimes even when introduced within therapeutic ranges (e.g. halothane), may injure the organ. Other chemical agents, such as those used in laboratories and industries, natural chemicals (e.g., microcystins), and herbal remedies (two prominent examples being kava, mechanism unknown, and comfrey, through its pyrrolizidine alkaloid content) can also induce hepatotoxicity. Chemicals that cause liver injury are called hepatotoxins. More than 900 drugs have been implicated in causing liver injury (see LiverTox, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Burroughs Wellcome
GSK plc, formerly GlaxoSmithKline plc, is a British multinational pharmaceutical and biotechnology company with global headquarters in London, England. Established in 2000 by a merger of Glaxo Wellcome and SmithKline Beecham. GSK is the tenth largest pharmaceutical company and #294 on the 2022 ''Fortune'' Global 500, ranked behind other pharmaceutical companies China Resources, Sinopharm, Johnson & Johnson, Pfizer, Roche, AbbVie, Novartis, Bayer, and Merck. The company has a primary listing on the London Stock Exchange and is a constituent of the FTSE 100 Index. , it had a market capitalisation of £70 billion, the eighth largest on the London Stock Exchange. It has a secondary listing on the New York Stock Exchange. The company developed the first malaria vaccine, RTS,S, which it said in 2014 it would make available for five percent above cost. Legacy products developed at GSK include several listed in the World Health Organization's List of Essential Medicines, such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nariman Mehta
Nariman Bomanshaw Mehta (April 20, 1920 – August 22, 2014) was an Indian-born American organic chemist and pharmacologist who designed, synthesized, and patented the organic compound bupropion, marketed under the name Wellbutrin as an antidepressant and smoking cessation aid. Early life and education Mehta was born in Bombay, India into a Parsi Zoroastrian family. He attended St. Xavier's College in Bombay, from where he received Bachelor of Science degrees in chemistry and physics and Bachelor of Arts degrees in English and economics, and a Master of Science degree. In 1939, he and fellow student Kaikhosrov D. Irani, later a noted academic in his own right, wrote and published the book "Textbook of Theoretical and Practical Physics". Mehta won a Tata Scholarship and received a grant from Wendell Willkie. In 1947 Mehta went to the United States, where he earned a PhD in chemistry from The University of Kansas. Mehta's 1952 dissertation was titled ''I. Use of the Hammett eq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Phenethylamine
Substituted phenethylamines (or simply phenethylamines) are a chemical class of organic compounds that are based upon the phenethylamine structure; the class is composed of all the derivative compounds of phenethylamine which can be formed by replacing, or substituting, one or more hydrogen atoms in the phenethylamine core structure with substituents. The structural formula of any substituted phenethylamine contains a phenyl ring that is joined to an amino (NH) group via a two-carbon sidechain. Hence, any substituted phenethylamine can be classified according to the substitution of hydrogen (H) atoms on phenethylamine's phenyl ring, sidechain, or amino group with a specific group of atoms. Many substituted phenethylamines are psychoactive drugs which belong to a variety of different drug classes, including central nervous system stimulants (e.g., amphetamine), hallucinogens (e.g., dl-2,5-dimethoxy-4-methylamphetamine DOM), entactogens (e.g., 3,4-methylenedioxyamphet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Amphetamine
Substituted amphetamines are a class of compounds based upon the amphetamine structure; it includes all derivative compounds which are formed by replacing, or substituting, one or more hydrogen atoms in the amphetamine core structure with substituents. The compounds in this class span a variety of pharmacological subclasses, including stimulants, empathogens, and hallucinogens, among others. Examples of substituted amphetamines are amphetamine (itself), methamphetamine, ephedrine, cathinone, phentermine, mephentermine, bupropion, methoxyphenamine, selegiline, amfepramone (diethylpropion), pyrovalerone, MDMA (ecstasy), and DOM (STP). Some of amphetamine's substituted derivatives occur in nature, for example in the leaves of '' Ephedra'' and khat plants. Amphetamine was first produced at the end of the 19th century. By the 1930s, amphetamine and some of its derivative compounds found use as decongestants in the symptomatic treatment of colds and also occasionally ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Cathinone
Substituted cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions. Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug. List of substituted cathinones The derivatives may be produced by substitutions at four locations of the cathinone molecule: * R1 = hydrogen, or any combination of one or more alkyl, alkoxy, alkylenedioxy, haloalkyl or halide substituents * R2 = hydrogen or any alkyl group * R3 = hydrogen, any alkyl group, or incorporation in a cyclic structure * R4 = hydrogen, any alkyl group, or incorporation in a cyclic structure The following table displays notable derivatives that have been reported: Legality On 2 April 2010, the Advisory Council on the Misuse of Drugs in the UK announced that a broad structure-based ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Classification
Chemical classification systems attempt to classify elements or compounds according to certain chemical functional or structural properties. Whereas the structural properties are largely intrinsic, functional properties and the derived classifications depend to a certain degree on the type of chemical interaction partners on which the function is exerted. Sometimes other criteria like purely physical ones (e.g. molecular weight) or - on the other hand - functional properties above the chemical level are also used for building chemical taxonomies. Some systems mix the various levels, resulting in hierarchies where the domains are slightly confused, for example having structural and functional aspects end up on the same level. Whereas chemical function is closely dependent on chemical structure, the situation becomes more involved when e.g. pharmacological function is integrated, because the QSAR can usually not be directly computed from structural qualities. Physico-chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminoaldehydes And Aminoketones
Aminoaldehydes and aminoketones are organic compounds that contain an amine functional group as well as either a aldehyde or ketone functional group. These compounds are important in biology and in chemical synthesis. Because of their bifunctional nature, they have attracted much attention from chemists. Tertiary amine derivatives Because primary and secondary amines react with aldehydes and ketones, the most common variety of these aminocarbonyl compounds feature tertiary amines. Such compounds are produced by amination of α-haloketones and α- haloaldehydes. Examples include cathinones, methadone, molindone, pimeclone, ferruginine, and tropinone. Secondary amine derivatives Aminoketones containing secondary amines are typically stable when the ketone is located on a ring, e.g. 4-piperidinone, triacetonamine, acridone Primary amine derivatives Most members of this class are unstable towards self-condensation, however some important examples do exist as intermediates in bi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. Dopamine constitutes about 80% of the catecholamine content in the brain. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor chemical, L-DOPA, which is synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons (nerve cells) to send signals to other nerve cells. Neurotransmitters are synthesized in specific regions of the brain, but affect many regions systemically. The brain includes several distinct dopamine pathways, one of which plays a major role in the motivational component of reward-motivated behavior. The anticipation of most types of rewards increases the level of dopamine in the brain, and ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinic Receptor Antagonist
A nicotinic antagonist is a type of anticholinergic drug that inhibits the action of acetylcholine (ACh) at nicotinic acetylcholine receptors. These compounds are mainly used for peripheral muscle paralysis in surgery, the classical agent of this type being tubocurarine,P. Taylor (1990). In ''Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th Ed.'', (A. G. Gilman et al., Eds.), pp. 166-186, New York: Pergamon Press. but some centrally acting compounds such as bupropion, mecamylamine, and 18-methoxycoronaridine block nicotinic acetylcholine receptors in the brain and have been proposed for treating nicotine addiction. *Note: Succinylcholine is a nicotinic agonist An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the ago .... See neuromuscular blocking agents page for det ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |