|
Anti-static
An antistatic agent is a compound used for treatment of materials or their surfaces in order to reduce or eliminate buildup of static electricity. Static charge may be generated by the triboelectric effect or by a non-contact process using a high voltage power source. Static charge may be introduced on a surface as part of an in-mold label printing process. The role of an antistatic agent is to make the surface or the material itself slightly conductive, either by being conductive itself, or by absorbing moisture from the air; therefore, some humectants can be used. The molecules of an antistatic agent often have both hydrophilic and hydrophobic areas, similar to those of a surfactant; the hydrophobic side interacts with the surface of the material, while the hydrophilic side interacts with the air moisture and binds the water molecules. Internal antistatic agents are designed to be mixed directly into the material, external antistatic agents are applied to the surface. Common a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Static Electricity
Static electricity is an imbalance of electric charges within or on the surface of a material or between materials. The charge remains until it is able to move away by means of an electric current or electrical discharge. Static electricity is named in contrast with current electricity, where the electric charge flows through an electrical conductor or space, and transmits energy. A static electric charge can be created whenever two surfaces contact and have worn and separated, and at least one of the surfaces has a high resistance to electric current (and is therefore an electrical insulator). The effects of static electricity are familiar to most people because people can feel, hear, and even see the spark as the excess charge is neutralized when brought close to a large electrical conductor (for example, a path to ground), or a region with an excess charge of the opposite polarity (positive or negative). The familiar phenomenon of a static shockmore specifically, an elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triboelectric Effect
The triboelectric effect (also known as triboelectric charging) is a type of contact electrification on which certain materials become electrically charged after they are separated from a different material with which they were in contact. Rubbing the two materials with each other increases the contact between their surfaces, and hence the triboelectric effect. Rubbing glass with fur for example, or a plastic comb through the hair, can build up triboelectricity. Most everyday static electricity is triboelectric. The polarity and strength of the charges produced differ according to the materials, surface roughness, temperature, strain, and other properties. The triboelectric effect is very unpredictable, and only broad generalizations can be made. Amber, for example, can acquire an electric charge by contact and separation (or friction) with a material like wool. This property was first recorded by Thales of Miletus. The word "electricity" is derived from William Gilbert's i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
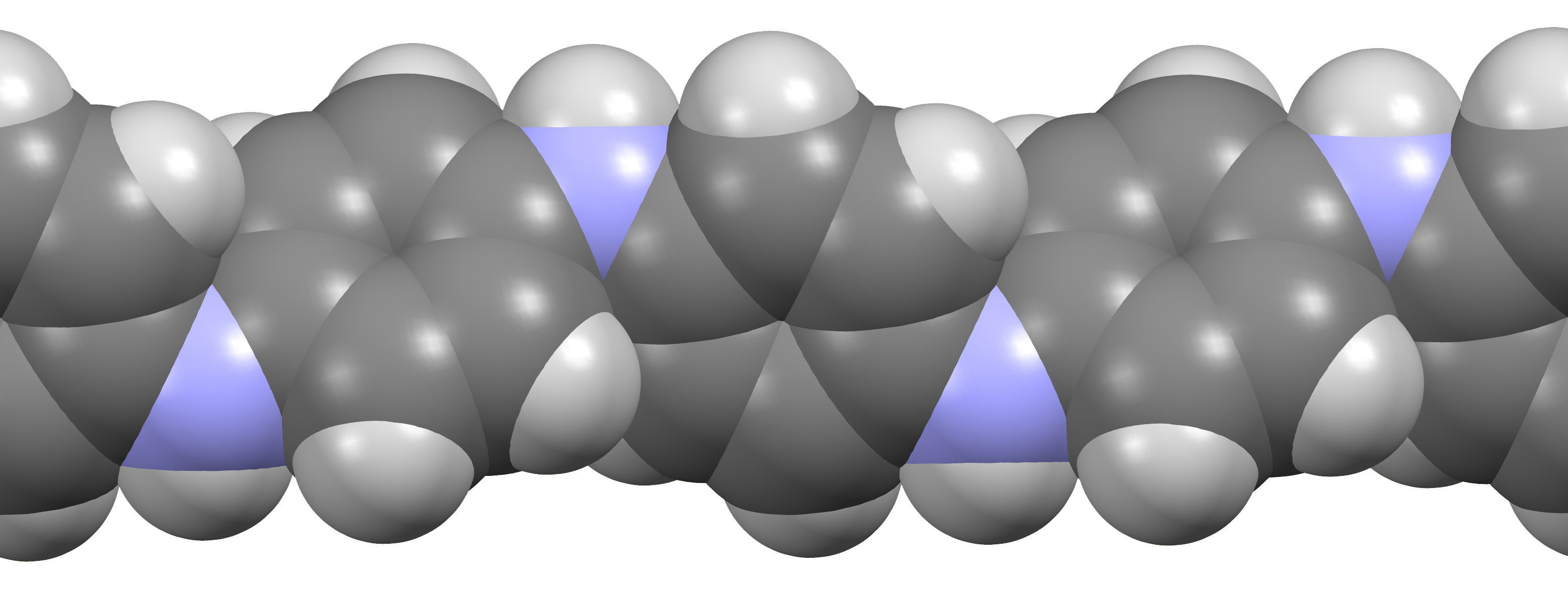
Dodecylbenzenesulfonic Acid
Alkylbenzene sulfonates are a class of anionic surfactants, consisting of a hydrophilic sulfonate head-group and a hydrophobic alkylbenzene tail-group. Along with sodium laureth sulfate, they are one of the oldest and most widely used synthetic detergents and may be found in numerous personal-care products (soaps, shampoos, toothpaste etc.) and household-care products (laundry detergent, dishwashing liquid, spray cleaner etc.).Kurt Kosswig,"Surfactants" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, 2005, Weinheim. They were first introduced in the 1930s in the form of branched alkylbenzene sulfonates (BAS). However following environmental concerns these were replaced with linear alkylbenzene sulfonates (LAS) during the 1960s. Since then production has increased significantly from about one million tons in 1980, to around 3.5 million tons in 2016, making them most produced anionic surfactant after soaps. __TOC__ Branched alkylbenzene sulfonates Branched alky ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonic Acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, , a tautomer of sulfurous acid, . Salt (chemistry), Salts or esters of sulfonic acids are called sulfonates. Preparation Aryl sulfonic acids are produced by the process of sulfonation. Usually the sulfonating agent is sulfur trioxide. A large scale application of this method is the production of alkylbenzenesulfonic acids: :RC6H5 + SO3 -> RC6H4SO3H In this reaction, sulfur trioxide is an electrophile and the arene is the nucleophile. The reaction is an example of electrophilic aromatic substitution. Alkyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JP-8
JP-8, or JP8 (for "Jet Propellant 8") is a jet fuel, specified and used widely by the US military. It is specified by MIL-DTL-83133 and British Defence Standard 91-87, and similar to commercial aviation's Jet A-1, but with the addition of corrosion inhibitor and anti-icing additives. A kerosene-based fuel, JP-8 is projected to remain in use at least until 2025. It was first introduced at NATO bases in 1978. Its NATO code is F-34''. Usage It was specified in 1990 by the U.S. government as a replacement for government diesel fueled vehicles. The U.S. Air Force replaced JP-4 with JP-8 completely by the end of 1995, to use a less flammable, less hazardous fuel for better safety and combat survivability. The U.S. Navy uses a similar formula, JP-5. JP-5 has an even higher flash point of > 140 °F (60 °C), but also a higher cost, though the U.S. Navy Seabees still use JP-8 in their construction and tactical equipment. In addition to its use for powering aircraft, JP-8 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distillate Fuel
Fuel oil is any of various fractions obtained from the distillation of petroleum (crude oil). Such oils include distillates (the lighter fractions) and residues (the heavier fractions). Fuel oils include heavy fuel oil, marine fuel oil (MFO), bunker fuel, furnace oil (FO), gas oil (gasoil), heating oils (such as home heating oil), diesel fuel and others. The term ''fuel oil'' generally includes any liquid fuel that is burned in a furnace or boiler to generate heat (heating oils), or used in an engine to generate power (as motor fuels). However, it does not usually include other liquid oils, such as those with a flash point of approximately , or oils burned in cotton- or wool-wick burners. In a stricter sense, ''fuel oil'' refers only to the heaviest commercial fuels that crude oil can yield, that is, those fuels heavier than gasoline (petrol) and naphtha. Fuel oil consists of long-chain hydrocarbons, particularly alkanes, cycloalkanes, and aromatics. Small molecules, such as thos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical synt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonpolar
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end. Polar molecules must contain one or more polar bonds due to a difference in electronegativity between the bonded atoms. Molecules containing polar bonds have no molecular polarity if the bond dipoles cancel each other out by symmetry. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points. Polarity of bonds Not all atoms attract electrons with the same force. The amount of "pull" an atom exerts on its electrons is called its electronegativity. Atoms with high electronegativitiessuch as fluorine, oxygen, and nitrogenexert a greater pull on electrons than atoms with lower electronegativities such as alkali metals and alka ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jet Fuel
Jet fuel or aviation turbine fuel (ATF, also abbreviated avtur) is a type of aviation fuel designed for use in aircraft powered by gas-turbine engines. It is colorless to straw-colored in appearance. The most commonly used fuels for commercial aviation are Jet A and Jet A-1, which are produced to a standardized international specification. The only other jet fuel commonly used in civilian turbine-engine powered aviation is Jet B, which is used for its enhanced cold-weather performance. Jet fuel is a mixture of a variety of hydrocarbons. Because the exact composition of jet fuel varies widely based on petroleum source, it is impossible to define jet fuel as a ratio of specific hydrocarbons. Jet fuel is therefore defined as a performance specification rather than a chemical compound. Furthermore, the range of molecular mass between hydrocarbons (or different carbon numbers) is defined by the requirements for the product, such as the freezing point or smoke point. Kerosene-type je ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanofiber
Nanofibers are fibers with diameters in the nanometer range (typically, between 1 nm and 1 μm). Nanofibers can be generated from different polymers and hence have different physical properties and application potentials. Examples of natural polymers include collagen, cellulose, silk fibroin, keratin, gelatin and polysaccharides such as chitosan and alginate. Examples of synthetic polymers include poly(lactic acid) (PLA), polycaprolactone (PCL), polyurethane (PU), poly(lactic-co-glycolic acid) (PLGA), poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), and poly(ethylene-co-vinylacetate) (PEVA). Polymer chains are connected via covalent bonds. The diameters of nanofibers depend on the type of polymer used and the method of production. All polymer nanofibers are unique for their large surface area-to-volume ratio, high porosity, appreciable mechanical strength, and flexibility in functionalization compared to their microfiber counterparts. There exist many different methods to m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyaniline
Polyaniline (PANI) is a conducting polymer and organic semiconductor of the semi-flexible rod polymer family. The compound has been of interest since the 1980s because of its electrical conductivity and mechanical properties. Polyaniline is one of the most studied conducting polymers. Historical development Polyaniline was discovered in the 19th century by F. Ferdinand Runge (1794–1867), Carl Fritzsche (1808–1871), John Lightfoot (1831–1872), and Henry Letheby (1816–1876). Lightfoot studied the oxidation of aniline, which had been isolated only 20 years previously. He developed the first commercially successful route to the dye called Aniline black. The first definitive report of polyaniline did not occur until 1862, which included an electrochemical method for the determination of small quantities of aniline. From the early 20th century on, occasional reports about the structure of PANI were published. Polymerized from the inexpensive aniline, polyaniline can be fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conductive Polymer
Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The biggest advantage of conductive polymers is their processability, mainly by dispersion. Conductive polymers are generally not thermoplastics, ''i.e.'', they are not thermoformable. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. The electrical properties can be fine-tuned using the methods of organic synthesis and by advanced dispersion techniques. History Polyaniline was first described in the mid-19th century by Henry Letheby, who investigated the electrochemical and chemical oxidation products of aniline in acidic media. He noted that reduced form was colourless but the oxidized forms were deep blue. The first highly-conductive org ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)