|
Aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine In organic chemistry, an aromatic amine is an organic compound consisting of an aromatic ring attached to an amine. It is a broad class of compounds that encompasses aniline Aniline is an organic compound with the formula C6 H5 NH2. Consi .... It is an industrially significant Commodity chemicals, commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It Combustion, ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, it is electron-rich. It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Group
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. Aromatic a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,4,6-Trinitroaniline
2,4,6-Trinitroaniline, C6H4N4O6, abbreviated as TNA and also known as picramide, a nitrated amine. Materials in this group range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups. The appearance of trinitroaniline varies from yellow to orange to red depending on its purity and concentration. Applications/Uses Trinitroaniline is only used in modern times in the small warheads of some explosive devices such as mortars. In World War II it was used by Imperial Japanese Navy as Type 97 ''bakuyaku'' (Model 1931 explosive) in some versions of gun projectiles instead of less stable burst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Amine
In organic chemistry, an aromatic amine is an organic compound consisting of an aromatic ring attached to an amine. It is a broad class of compounds that encompasses anilines, but also many more complex aromatic rings and many amine substituents beyond . Such compounds occur widely. Aromatic amines are widely used as precursor to pesticides, pharmaceuticals, and dyes. Aromatic amines in textiles Since August 2012, the new standard EN 14362-1:2012 ''Textiles - Methods for determination of certain aromatic amines derived from azo colorants - Part 1: Detection of the use of certain azo colorants accessible with and without extracting the fibres'' is effective. It had been officially approved by the European Committee for Standardization (CEN) and supersedes the test standards EN 14362-1: 2003 and EN 14362-2: 2003. The standard describes a procedure to detect EU banned aromatic amines derived from azo colorants in textile fibres, including natural, man-made, regenerated, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazonium Compound
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. General properties and reactivity Arenediazonium cations and related species According to X-ray crystallography the linkage is linear in typical diazonium salts. The bond distance in benzenediazonium tetrafluoroborate is 1.083(3) Å, which is almost identical to that for dinitrogen molecule (N≡N). The linear free energy constants σm and σp indicate that the diazonium group is strongly electron-withdrawing. Thus, the diazonio-substituted phenols and benzoic acids have greatly reduced p''K''a values compared to their unsubstituted counterparts. The p''K''a of phenolic proton of 4-hydroxybenzenediazonium is 3.4, versus 9.9 for phenol itself. In other words, the diazonium group lowers the p''K''a (enhances the acidity) by a million-fold. The stabi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents. Production Nitrobenzene is prepared by nitration of benzene with a mixture of concentrated sulfuric acid, water, and nitric acid. This mixture is sometimes called "mixed acid." The production of nitrobenzene is one of the most dangerous processes conducted in the chemical industry because of the exothermicity of the reaction (Δ''H'' = −117 kJ/mol). World capacity for nitrobenzene in 1985 was about 1,700,000 tonnes. The nitration process involves formation of the nitronium ion (NO2+), followed by an electrophilic aromatic substitution reaction of it with benzene. The nitronium ion is generated by the reaction of nitric aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrosobenzene
Nitrosobenzene is the organic compound with the formula C6H5NO. It is one of the prototypical organic nitroso compounds. Characteristic of its functional group, it is a dark green species that exists in equilibrium with its pale yellow dimer. Both monomer and dimer are diamagnetic. Monomer-dimer equilibrium Nitrosobenzene and other nitrosoarenes typically participate in a monomer-dimer equilibrium. The dimers are often favored in the solid state, whereas the deeply colored monomers are favored in dilute solution or at higher temperatures. The dimers can be formulated as Ar(O−)N+=N+(O−)Ar. They exist as ''cis''- and ''trans''-isomers due to the presence of the N–N double bond. The dimers are sometimes called azobenzenedioxides. The cis-trans isomerization occurs via the intermediacy of the monomer. In the case of nitrosobenzene itself, the metastable monomeric form could be prepared by sublimation onto a cold finger. The monomeric material is selectively sublimed due to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-Naphthylamine
1-Naphthylamine is an aromatic amine derived from naphthalene. It can cause bladder cancer (transitional cell carcinoma). It crystallizes in colorless needles which melt at 50 °C. It possesses a disagreeable odor, sublimes readily, and turns brown on exposure to air. It is the precursor to a variety of dyes.. Preparation and reactions It can be prepared by reducing 1-nitronaphthalene with iron and hydrochloric acid followed by steam distillation. Oxidizing agents, such as ferric chloride, give a blue precipitate with solutions of its salts. Chromic acid converts it into 1-naphthoquinone. Sodium in boiling amyl alcohol reduces the unsubstituted ring, giving tetrahydro-1-naphthylamine. This tetrahydro compound yields adipic acid when oxidized by potassium permanganate. At 200 °C in sulfuric acid, it converts to 1-naphthol. Use in dyes The sulfonic acid derivatives of 1-naphthylamine are used for the preparation of azo dye. These compounds possess the important pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
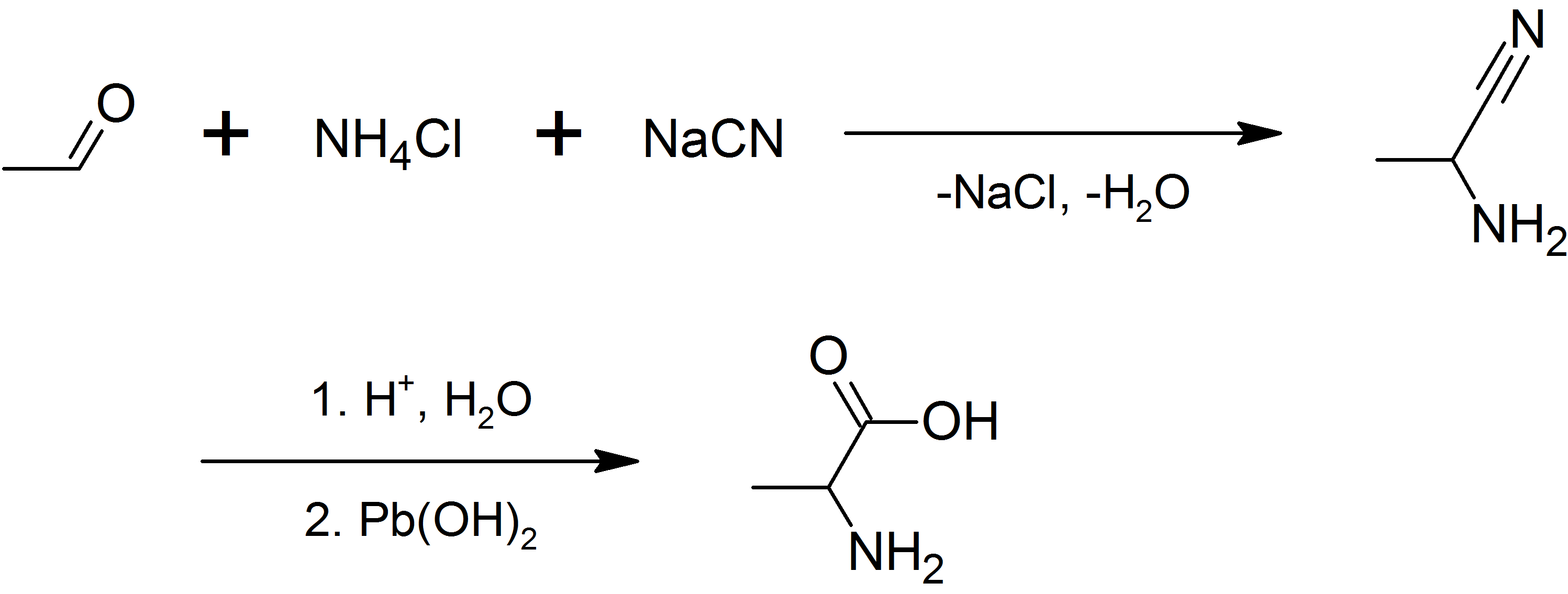
Alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG). The L-isomer of alanine (left-handed) is the one that is incorporated into proteins. L-alanine is second only to leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The right-handed form, D-alanine, occurs in p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pascal Second
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity quantifies the internal frictional force between adjacent layers of fluid that are in relative motion. For instance, when a viscous fluid is forced through a tube, it flows more quickly near the tube's axis than near its walls. Experiments show that some stress (such as a pressure difference between the two ends of the tube) is needed to sustain the flow. This is because a force is required to overcome the friction between the layers of the fluid which are in relative motion. For a tube with a constant rate of flow, the strength of the compensating force is proportional to the fluid's viscosity. In general, viscosity depends on a fluid's state, such as its temperature, pressure, and rate of deformation. However, the dependence on some of these properties is n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fine Chemical
In chemistry, fine chemicals are complex, single, pure chemical substances, produced in limited quantities in multipurpose plants by multistep batch chemical or biotechnological processes. They are described by exacting specifications, used for further processing within the chemical industry and sold for more than $10/kg (see the comparison of fine chemicals, commodities and specialties). The class of fine chemicals is subdivided either on the basis of the added value (building blocks, advanced intermediates or active ingredients), or the type of business transaction, namely standard or exclusive products. Fine chemicals are produced in limited volumes ( $10/kg) according to exacting specifications, mainly by traditional organic synthesis in multipurpose chemical plants. Biotechnical processes are gaining ground. Fine chemicals are used as starting materials for specialty chemicals, particularly pharmaceuticals, biopharmaceuticals and agrochemicals. Custom manufacturing f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituent Effect
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side chain'' and ''pendant group'', are used almost interchangeably to describe those branches from the parent structure, though certain distinctions are made in polymer chemistry. In polymers, side chains extend from the backbone structure. In proteins, side chains are attached to the alpha carbon atoms of the amino acid backbone.) The suffix ''-yl'' is used when naming organic compounds that contain a single bond replacing one hydrogen; ''-ylidene'' and ''-ylidyne'' are used with double bonds and triple bonds, respectively. In addition, when naming hydrocarbons that contain a substituent, positional numbers are used to indicate which carbon atom the substituent attaches to when such information is needed to distinguish between isomers. Subs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |