|
Amylocaine
Amylocaine was the first synthetic local anesthetic. It was synthesized and patented under the name Stovaine by Ernest Fourneau at the Pasteur Institute in 1903. It was used mostly in spinal anesthesia.Debue-Barazer, Christine (2007)"Les Implications scientifiques et industrielles du succès de la Stovaïne : Ernest Fourneau (1872-1949) et la chimie des médicaments en France". ''Gesnerus'' 64 (1-2): 24-53. Synthesis Grignard reaction of chloroacetone Chloroacetone is a chemical compound with the chemical formula, formula . At Standard conditions for temperature and pressure, STP it is a colourless liquid with a pungent odour. On exposure to light, it turns to a dark yellow-amber colour. It wa ... (1) with one mole of magnesium ethyl bromide gives 1-chloro-2-methyl-butan-2-ol 4283-48-0(2). Heating with dimethylamine gives 1-(dimethylamino)-2-methylbutan-2-ol 4347-10-7(3). These two steps can also be treated as interchangeable. Esterification with benzoyl chloride completed the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ernest Fourneau
Ernest Fourneau (4 October 1872 – 5 August 1949) was a French pharmacist graduated in Pharmacy 1898 for the Paris university specialist in medicinal chemical and pharmacology who played a major role in the discovery of synthetic local anesthetics, as well as in the synthesis of suramin. He authored more than two hundred scholarly works, and has been described as having "helped to establish the fundamental laws of chemotherapy that have saved so many human lives". Fourneau was a pupil of Friedel and Moureu, and studied in the German laboratories of Ludwig Gattermann in Heidelberg, Hermann Emil Fischer in Berlin and Richard Willstätter in Munich. He headed the research laboratory of Poulenc Frères in Ivry-sur-Seine from 1903 to 1911. One of the products was a synthetic local anesthetic that was named Stovaine (amylocaine). This was a pun on the English translation of "fourneau" as "stove". (The same pun was used in the brand name of the drug acetarsol, Stovarsol.) Other im ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amylocaine Synthesis
Amylocaine was the first synthetic local anesthetic. It was synthesized and patented under the name Stovaine by Ernest Fourneau at the Pasteur Institute in 1903. It was used mostly in spinal anesthesia.Debue-Barazer, Christine (2007)"Les Implications scientifiques et industrielles du succès de la Stovaïne : Ernest Fourneau (1872-1949) et la chimie des médicaments en France". ''Gesnerus'' 64 (1-2): 24-53. Synthesis Grignard reaction of chloroacetone Chloroacetone is a chemical compound with the chemical formula, formula . At Standard conditions for temperature and pressure, STP it is a colourless liquid with a pungent odour. On exposure to light, it turns to a dark yellow-amber colour. It wa ... (1) with one mole of magnesium ethyl bromide gives 1-chloro-2-methyl-butan-2-ol 4283-48-0(2). Heating with dimethylamine gives 1-(dimethylamino)-2-methylbutan-2-ol 4347-10-7(3). These two steps can also be treated as interchangeable. Esterification with benzoyl chloride completed the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Local Anesthetic
A local anesthetic (LA) is a medication that causes absence of pain sensation. In the context of surgery, a local anesthetic creates an absence of pain in a specific location of the body without a loss of consciousness, as opposed to a general anesthetic. When it is used on specific nerve pathways ( local anesthetic nerve block), paralysis (loss of muscle power) also can be achieved. Examples Short Duration & Low Potency Procaine Chloroprocaine Medium Duration & Potency Lidocaine Prilocaine High Duration & Potency Tetracaine Bupivacaine Cinchocaine Ropivacaine Clinical LAs belong to one of two classes: aminoamide and aminoester local anesthetics. Synthetic LAs are structurally related to cocaine. They differ from cocaine mainly in that they have a very low abuse potential and do not produce hypertension or (with few exceptions) vasoconstriction. They are used in various techniques of local anesthesia such as: * Topical anesthesia (surface) * Topical ad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pasteur Institute
The Pasteur Institute (french: Institut Pasteur) is a French non-profit private foundation dedicated to the study of biology, micro-organisms, diseases, and vaccines. It is named after Louis Pasteur, who invented pasteurization and vaccines for anthrax and rabies. The institute was founded on 4 June 1887, and inaugurated on 14 November 1888. For over a century, the Institut Pasteur has researched infectious diseases. This worldwide biomedical research organization based in Paris was the first to isolate HIV, the virus that causes AIDS, in 1983. Over the years, it has been responsible for discoveries that have enabled medical science to control diseases such as diphtheria, tetanus, tuberculosis, poliomyelitis, influenza, yellow fever, and plague. Since 1908, ten Institut Pasteur scientists have been awarded the Nobel Prize for medicine and physiology—the 2008 Nobel Prize in Physiology or Medicine was shared between two Pasteur scientists. History The Institut Pas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
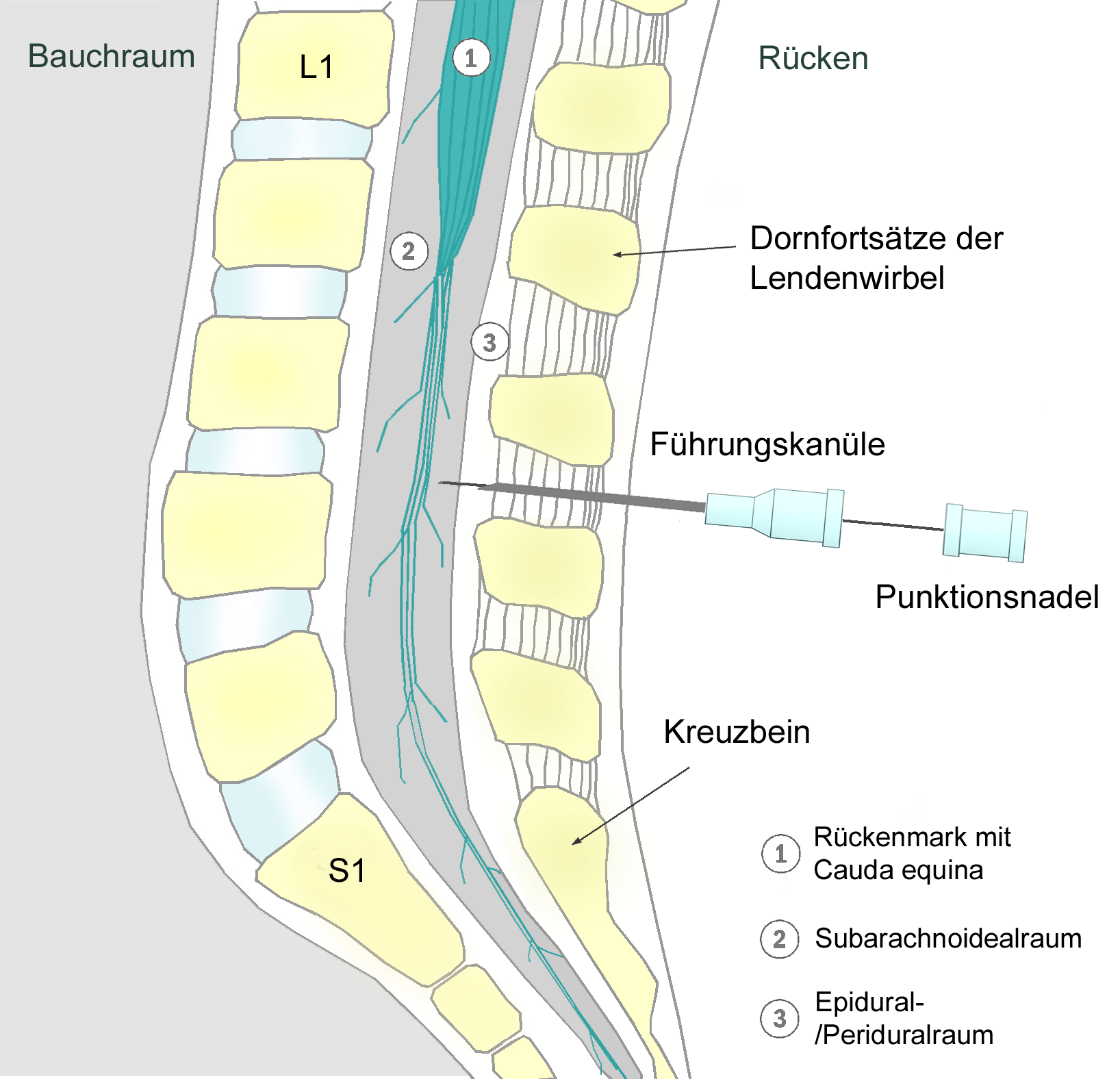
Spinal Anesthesia
Spinal anaesthesia (or spinal anesthesia), also called spinal block, subarachnoid block, intradural block and intrathecal block, is a form of neuraxial regional anaesthesia involving the injection of a local anaesthetic or opioid into the subarachnoid space, generally through a fine needle, usually long. It is a safe and effective form of anesthesia usually performed by anesthesiologists that can be used as an alternative to general anesthesia commonly in surgeries involving the lower extremities and surgeries below the umbilicus. The local anesthetic with or without an opioid injected into the cerebrospinal fluid provides locoregional anaesthesia: true analgesia, motor, sensory and autonomic (sympathic) blockade. Administering analgesics (opioid, alpha2-adrenoreceptor agonist) in the cerebrospinal fluid without a local anaesthetic produces locoregional analgesia: markedly reduced pain sensation (incomplete analgesia), some autonomic blockade (parasympathetic plexi), but no se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reaction
The Grignard reaction () is an organometallic chemical reaction in which alkyl, allyl, vinyl, or aryl-magnesium halides (Grignard reagent) is added to a carbonyl group in an aldehyde or ketone. This reaction is important for the formation of carbon–carbon bonds. The reaction of an organic halide with magnesium is ''not'' a Grignard reaction, but provides a Grignard reagent. : Grignard reactions and reagents were discovered by and are named after the French chemist François Auguste Victor Grignard ( University of Nancy, France), who published it in 1900 and was awarded the 1912 Nobel Prize in Chemistry for this work. Reaction mechanism Because carbon is more electronegative than magnesium, the carbon attached to magnesium functions as a nucleophile and attacks the electrophilic carbon atom that is present within the polar bond of a carbonyl group. The addition of the Grignard reagent to the carbonyl typically proceeds through a six-membered ring transition state. Based ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroacetone
Chloroacetone is a chemical compound with the chemical formula, formula . At Standard conditions for temperature and pressure, STP it is a colourless liquid with a pungent odour. On exposure to light, it turns to a dark yellow-amber colour. It was used as a lachrymatory agent, tear gas in World War I. Synthesis Chloroacetone may be synthesized from the reaction between chlorine and diketene, or by the chlorination of acetone. Applications Chloroacetone is used to make dye couplers for colour photography, and is an intermediate in chemical manufacturing. It is also used in the Feist-Benary synthesis of furans. *Reaction of phenoxide with chloroacetone gives phenoxyacetone, which is used to make a wide variety of different pharmaceuticals. A catalytic amount of potassium iodide is also necessary to facilitate a Finkelstein reaction. Purification Chloroacetone purchased from commercial suppliers contains 5% impurities including mesityl oxide, which is not removed by distillatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylaminopivalophenone
Dimethylaminopivalophenone is an opioid analgesic with a potency ½ that of morphine. It was initially discovered by Russian scientists in 1954 and subsequently rediscovered in the US in 1969. Its LD50 in mice is 83 mg/kg. It has never been marketed commercially. See also * Tapentadol * List of opioids This is a list of opioids, opioid antagonists and inverse agonists. Opium and poppy straw derivatives Crude opiate extracts whole opium products * B&O Supprettes * Diascordium *Dover's powder * Kendal Black Drop *Laudanum *Mithridate *Op ... * Opioid#Table of non-morphinan opioids References Mu-opioid receptor agonists Aromatic ketones Dimethylamino compounds {{analgesic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structure–activity Relationship
The structure–activity relationship (SAR) is the relationship between the chemical structure of a molecule and its biological activity. This idea was first presented by Crum-Brown and Fraser in 1865. The analysis of SAR enables the determination of the chemical group responsible for evoking a target biological effect in the organism. This allows modification of the effect or the potency of a bioactive compound (typically a drug) by changing its chemical structure. Medicinal chemists use the techniques of chemical synthesis to insert new chemical groups into the biomedical compound and test the modifications for their biological effects. This method was refined to build mathematical relationships between the chemical structure and the biological activity, known as quantitative structure–activity relationships (QSAR). A related term is structure affinity relationship (SAFIR). Structure-biodegradability relationship The large number of synthetic organic chemicals currently in p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylene Spacer
In organic chemistry, a methylene bridge, methylene spacer, or methanediyl group is any part of a molecule with formula ; namely, a carbon atom bound to two hydrogen atoms and connected by single bonds to two other distinct atoms in the rest of the molecule. It is the repeating unit in the skeleton of the unbranched alkanes. A methylene bridge can also act as a bidentate ligand joining two metals in a coordination compound, such as titanium and aluminum in Tebbe's reagent.W. A. Herrmann (1982), "The methylene bridge". In ''Advances in Organometallic Chemistry'', volume 20, pages 195-197. A methylene bridge is often called a methylene group or simply methylene, as in "methylene chloride" ( dichloromethane ). As a bridge in other compounds, for example in cyclic compounds, it is given the name methano. However, the term methylene group (or "methylidene") properly applies to the group when it is connected to the rest of the molecule by a double bond (), giving it chemical pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Local Anesthetics
A local anesthetic (LA) is a medication that causes absence of pain sensation. In the context of surgery, a local anesthetic creates an absence of pain in a specific location of the body without a loss of consciousness, as opposed to a general anesthetic. When it is used on specific nerve pathways ( local anesthetic nerve block), paralysis (loss of muscle power) also can be achieved. Examples Short Duration & Low Potency Procaine Chloroprocaine Medium Duration & Potency Lidocaine Prilocaine High Duration & Potency Tetracaine Bupivacaine Cinchocaine Ropivacaine Clinical LAs belong to one of two classes: aminoamide and aminoester local anesthetics. Synthetic LAs are structurally related to cocaine. They differ from cocaine mainly in that they have a very low abuse potential and do not produce hypertension or (with few exceptions) vasoconstriction. They are used in various techniques of local anesthesia such as: * Topical anesthesia (surface) * Topical admi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoate Esters
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source. Benzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of benzoic acid are used as food preservatives. Benzoic acid is an important precursor for the industrial synthesis of many other organic substances. The salts and esters of benzoic acid are known as benzoates . History Benzoic acid was discovered in the sixteenth century. The dry distillation of gum benzoin was first described by Nostradamus (1556), and then by Alexius Pedemontanus (1560) and Blaise de Vigenère (1596). Justus von Liebig and Friedrich Wöhler determined the composition of benzoic acid. These latter also investigated how hippuric acid is rela ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |