|
Alizarin Red S
Alizarin Red S (also known as Colour Index International, C.I. Mordant Red 3, Alizarin Carmine, and C.I 58005.) is a water-soluble sodium salt (chemistry), salt of Alizarin sulfonic acid with a chemical formula of . Alizarin Red S was discovered by Carl Gräbe, Graebe and Carl Theodore Liebermann, Libermann in 1871. In the field of histology alizarin Red S is used to staining, stain calcium deposits in tissues, and in geology to stain and differentiate carbonate minerals. Uses Alizarin Red S is used in histology and histopathology to stain, or locate calcium deposits in tissues. In the presence of calcium, Alizarin Red S, binds to the calcium to form a Lake pigment that is orange to red in color. Whole specimens can be stained with Alizarin Red S to show the distribution of bone, especially in developing embryos. In living corals alizarin Red S has been used to mark daily growth layers. In geology, Alizarin Red S is used on Thin sections, and polished surfaces to help identify c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colour Index International
Colour Index International is a reference database jointly maintained by the Society of Dyers and Colourists and the American Association of Textile Chemists and Colorists. It currently contains over 27,000 individual products listed under 13,000 Colour Index Generic Names. It was first printed in 1925 but is now published solely on the World Wide Web. The index serves as a common reference database of manufactured colour products and is used by manufacturers and consumers, such as artists and decorators. Colorants (both dyes and pigments) are listed using a dual classification which use the Colour Index Generic Name (the prime identifier) and Colour Index Constitution Numbers. These numbers are prefixed with C.I. or CI, for example, C.I. 15510. (This abbreviation is sometimes mistakenly thought to be CL, due to the font used to display it.) A detailed record of products available on the market is presented under each Colour Index reference. For each product name, Colour Index I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine In organic chemistry, an aromatic amine is an organic compound consisting of an aromatic ring attached to an amine. It is a broad class of compounds that encompasses aniline Aniline is an organic compound with the formula C6 H5 NH2. Consi .... It is an industrially significant Commodity chemicals, commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It Combustion, ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, it is electron-rich. It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
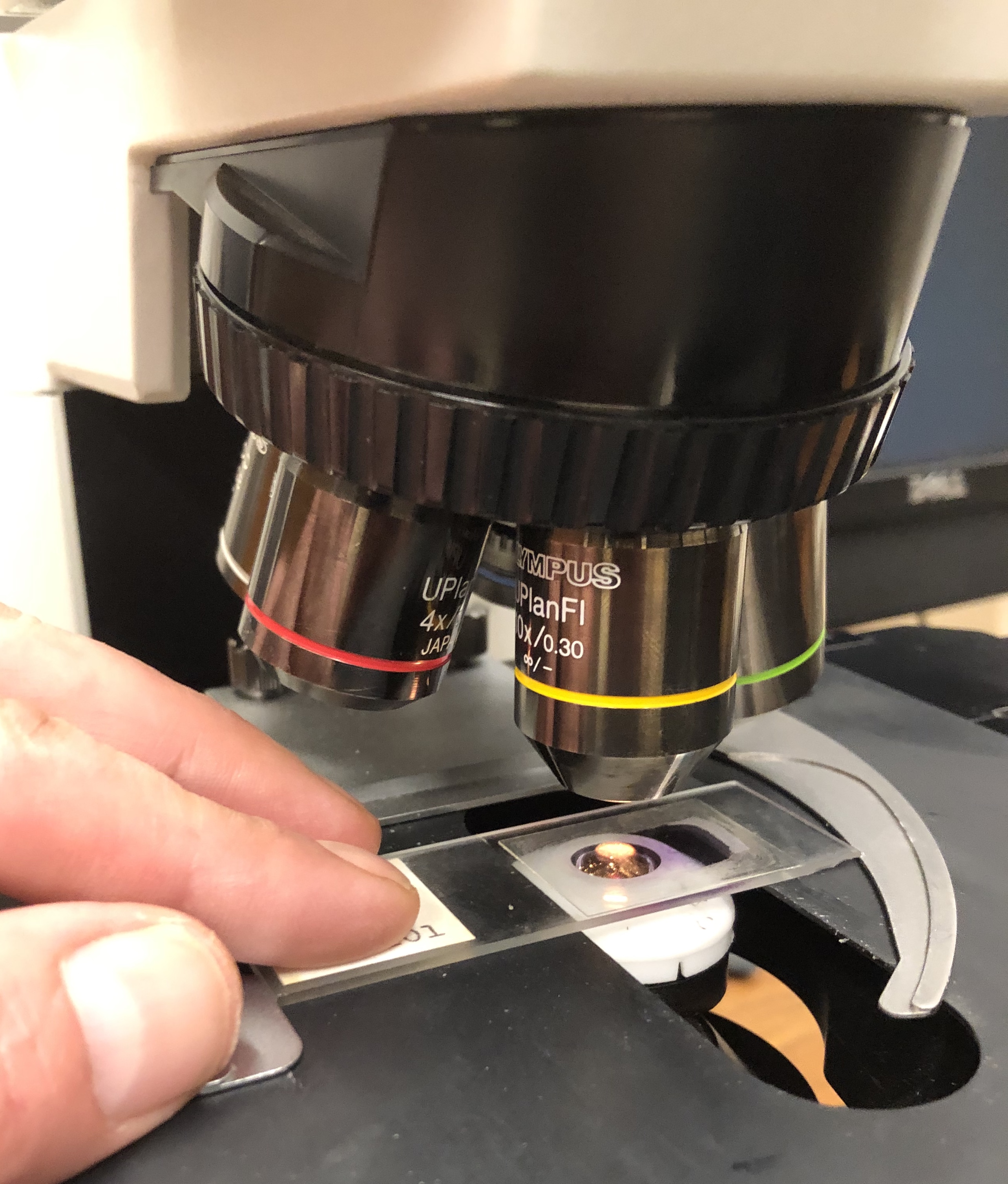
Histology
Histology, also known as microscopic anatomy or microanatomy, is the branch of biology which studies the microscopic anatomy of biological tissues. Histology is the microscopic counterpart to gross anatomy, which looks at larger structures visible without a microscope. Although one may divide microscopic anatomy into ''organology'', the study of organs, ''histology'', the study of tissues, and ''cytology'', the study of cells, modern usage places all of these topics under the field of histology. In medicine, histopathology is the branch of histology that includes the microscopic identification and study of diseased tissue. In the field of paleontology, the term paleohistology refers to the histology of fossil organisms. Biological tissues Animal tissue classification There are four basic types of animal tissues: muscle tissue, nervous tissue, connective tissue, and epithelial tissue. All animal tissues are considered to be subtypes of these four principal tissue types ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Staining Dyes
Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology (microscopic study of biological tissues), in cytology (microscopic study of cells), and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues (highlighting, for example, muscle fibers or connective tissue), cell populations (classifying different blood cells), or organelles within individual cells. In biochemistry, it involves adding a class-specific ( DNA, proteins, lipids, carbohydrates) dye to a substrate to qualify or quantify the presence of a specific compound. Staining and fluorescent tagging can serve similar purposes. Biological staining is also used to mark cells in flow cytometry, and to flag proteins or nucleic acids in gel electrophoresis. Light microscopes are used for viewing st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Dyes
Natural dyes are dyes or colorants derived from plants, invertebrates, or minerals. The majority of natural dyes are vegetable dyes from plant sources—roots, berries, bark, leaves, and wood—and other biological sources such as fungi. Archaeologists have found evidence of textile dyeing dating back to the Neolithic period. In China, dyeing with plants, barks and insects has been traced back more than 5,000 years.Goodwin (1982), p. 11. The essential process of dyeing changed little over time. Typically, the dye material is put in a pot of water and heated to extract the dye compounds into solution with the water. Then the textiles to be dyed are added to the pot, and held at heat until the desired color is achieved. Textile fibre may be dyed before spinning or weaving ("dyed in the wool"), after spinning ("yarn-dyed") or after weaving ("piece-dyed"). Many natural dyes require the use of substances called mordants to bind the dye to the textile fibres. Mordants (from the Latin ver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Pigments
Organic may refer to: * Organic, of or relating to an organism, a living entity * Organic, of or relating to an anatomical organ Chemistry * Organic matter, matter that has come from a once-living organism, is capable of decay or is the product of decay, or is composed of organic compounds * Organic compound, a compound that contains carbon ** Organic chemistry, chemistry involving organic compounds Farming, certification and products * Organic farming, agriculture conducted according to certain standards, especially the use of stated methods of fertilization and pest control * Organic certification, accreditation process for producers of organically-farmed products * Organic horticulture, the science and art of growing fruits, vegetables, flowers, or ornamental plants by following the essential principles of organic agriculture * Organic products, "organics": ** Organic food, food produced from organic farming methods and often certified organic according to organic farming sta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydroxyanthraquinones
A dihydroxyanthraquinone is any of several isomeric organic compounds with formula , formally derived from 9,10-anthraquinone by replacing two hydrogen atoms by hydroxyl groups. Dihyroxyantraquinones have been studied since the early 1900s, and include some compounds of historical and current importance. The isomers differ in the position of the hydroxyl groups, and of the carbonyl oxygens (=O) of the underlying anthraquinone. Isomers From 9,10-anthraquinone The unqualified term "dihydroxyanthraquinone" usually means a hydroxy derivative of 9,10-anthraquinone. The dihydroxy-9,10-anthraquinone functional group occurs widely in natural products, and is an important feature of the anthracycline antitumour antibiotics. In particular, 1,8-Dihydroxy-9,10-anthraquinone is the precursor for the important topical antipsoriatic drug anthralin, 1,8-dihydroxy-9-anthrone, There are 28 ways of choosing two of the 8 possible hydrogens, but because of the four-fold symmetry of the 9,10-anth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelating Agents
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease. Chelation is useful in applications such as providing nutritional supplements, in chelation therapy to remove toxic metals from the body, as contrast agents in MRI scanning, in manufacturing using homogeneous catalysts, in chemical water treatment to assist in the removal of metals, and in fertilizers. Chelate effect The chelate effect is the greater affinity of chelating ligands for a metal ion than that of similar nonchelating (monodentate) ligands for the same metal. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catechols
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. It was first discovered by destructive distillation of the plant extract catechin. About 20,000 tonnes of catechol are now synthetically produced annually as a commodity organic chemical, mainly as a precursor to pesticides, flavors, and fragrances. Catechol occurs as feathery white crystals that are very rapidly soluble in water. Isolation and synthesis Catechol was first isolated in 1839 by Edgar Hugo Emil Reinsch (1809–1884) by distilling it from the solid tannic preparation catechin, which is the residuum of catechu, the boiled or concentrated juice of ''Mimosa catechu'' (''Acacia catechu''). Upon heating catechin above its decomposition point, a substance that Reinsch first named ''Brenz-Katechusäure'' (burned catechu acid) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthraquinone Dyes
Anthraquinone dyes are an abundant group of dyes comprising a anthraquinone unit as the shared structural element. Anthraquinone itself is colourless, but red to blue dyes are obtained by introducing electron donor groups such as hydroxy or amino groups in the 1-, 4-, 5- or 8-position. Anthraquinone dyestuffs are structurally related to indigo dyestuffs and are classified together with these in the group of carbonyl dyes. Members of this dye group can be found in natural dyes as well as in synthetic dyes. Anthraquinone dyestuffs are represented in mordant and vat, but also in reactive and disperse dyes. They are characterized by very good light fastness. Natural anthraquinone dyes One of the most important anthraquinone dyes of herbal origin is alizarin, which is extracted from the dyer's madder ( Rubia tinctorum). Alizarin is the eponym for a number of structurally related dyes that use alizarin dyes (sometimes synonymous with anthraquinone dyes). It was the first natural ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Colors (compact)
The following list shows a compact version of the colors in the list of colors A–F, G–M, and N–Z articles. The list shows the color swatch and its name. Hovering over the color box shows the HSV, RGB, and #hex values for the color in the tool tip. All values and conversions are in the sRGB color space, which is an inappropriate assumption for some entries. List of colors A B C D E F G–H I–K L M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Dyes
This is a list of dyes with Colour Index International generic names and numbers and CAS Registry Number, CAS Registry numbers. Note * Synonyms should be treated with caution because they are often used inconsistently, see Talk:List_of_dyes, discussion page and external lin See also * Glossary of dyeing terms Sources * BDH laboratory chemicals & biochemicals catalogue 1983 * Important Early Synthetic Dyes 1991 Smithsonian Institute External links Stainsfile dye indexWorld Dye VarietyHazardous substances used in textile dyeing Dyes Chemistry-related lists, Dyes {{dyeing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |