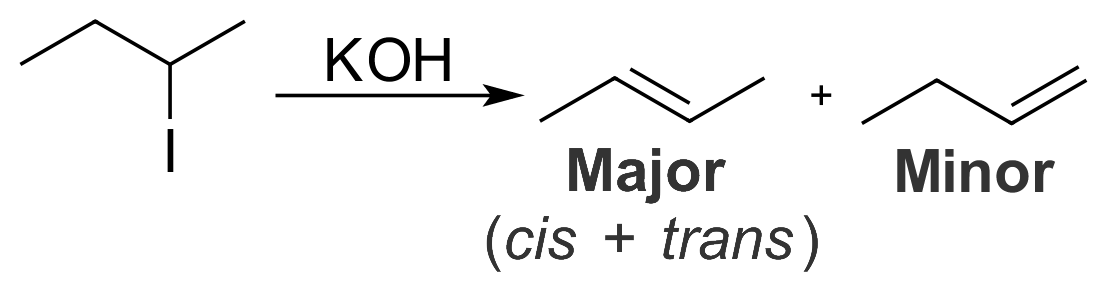|
Alexander Zaytsev (chemist)
Aleksander Mikhaylovich Zaytsev, also Zaitsev, Saytzeff, or Saytzev (russian: Алекса́ндр Миха́йлович За́йцев; 2 July 1841 – 1 September 1910) was a Russian chemist. He worked on organic compounds and proposed Zaytsev's rule, which predicts the product composition of an elimination reaction. Early years Zaytsev was born in Kazan. He was the son of a tea and sugar merchant, who had decided that his son should follow him into the mercantile trades.Lewis, D.E"Aleksandr Mikhailovich Zaitsev: Markovnikov's Conservative Contemporary."''Bull. Hist. Chem.'' 1995, ''17/18'', 21–30. However, at the urging of his maternal uncle, Zaytsev was allowed to enroll at University of Kazan to study economics. At this time, Russia was experimenting with the cameral system, meaning that every student graduating in law and economics from a Russian university had to take two years of chemistry. Zaytsev was thus introduced to Aleksandr Mikhailovich Butlerov. Earl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alexander Mikhaylovich Zaytsev
Aleksander Mikhaylovich Zaytsev (russian: Алекса́ндр Миха́йлович За́йцев), also spelled as Saytzeff and Saytzev (2 July 1841 – 1 September 1910), was a Russian chemist. He worked on organic compounds and proposed Zaitsev's rule, which predicts the product composition of an elimination reaction. Early years Zaytsev was born in Kazan. He was the son of a tea and sugar merchant, who had decided that his son should follow him into the mercantile trades.Lewis, D.E"Aleksandr Mikhailovich Zaitsev: Markovnikov's Conservative Contemporary."'' Bull. Hist. Chem.'' 1995, ''17/18'', 21–30. However, at the urging of his maternal uncle, Zaytsev was allowed to enroll at University of Kazan to study economics. At this time, Russia was experimenting with the cameral system, meaning that every student graduating in law and economics from a Russian university had to take two years of chemistry. Zaytsev was thus introduced to Aleksandr Mikhailovich Butlerov. Early ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leipzig
Leipzig ( , ; Upper Saxon: ) is the most populous city in the German state of Saxony. Leipzig's population of 605,407 inhabitants (1.1 million in the larger urban zone) as of 2021 places the city as Germany's eighth most populous, as well as the second most populous city in the area of the former East Germany after (East) Berlin. Together with Halle (Saale), the city forms the polycentric Leipzig-Halle Conurbation. Between the two cities (in Schkeuditz) lies Leipzig/Halle Airport. Leipzig is located about southwest of Berlin, in the southernmost part of the North German Plain (known as Leipzig Bay), at the confluence of the White Elster River (progression: ) and two of its tributaries: the Pleiße and the Parthe. The name of the city and those of many of its boroughs are of Slavic origin. Leipzig has been a trade city since at least the time of the Holy Roman Empire. The city sits at the intersection of the Via Regia and the Via Imperii, two important medieval trad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Russian Academy Of Science
The Russian Academy of Sciences (RAS; russian: Росси́йская акаде́мия нау́к (РАН) ''Rossíyskaya akadémiya naúk'') consists of the national academy of Russia; a network of scientific research institutes from across the Russian Federation; and additional scientific and social units such as libraries, publishing units, and hospitals. Peter the Great established the Academy (then the St. Petersburg Academy of Sciences) in 1724 with guidance from Gottfried Leibniz. From its establishment, the Academy benefitted from a slate of foreign scholars as professors; the Academy then gained its first clear set of goals from the 1747 Charter. The Academy functioned as a university and research center throughout the mid-18th century until the university was dissolved, leaving research as the main pillar of the institution. The rest of the 18th century continuing on through the 19th century consisted of many published academic works from Academy scholars and a few Ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moscow University
M. V. Lomonosov Moscow State University (MSU; russian: Московский государственный университет имени М. В. Ломоносова) is a public research university in Moscow, Russia and the most prestigious university in the country. The university includes 15 research institutes, 43 faculties, more than 300 departments, and six branches (including five foreign ones in the Commonwealth of Independent States countries). Alumni of the university include past leaders of the Soviet Union and other governments. As of 2019, 13 Nobel laureates, six Fields Medal winners, and one Turing Award winner had been affiliated with the university. The university was ranked 18th by ''The Three University Missions Ranking'' in 2022, and 76th by the ''QS World University Rankings'' in 2022, #293 in the world by the global ''Times Higher World University Rankings'', and #326 by '' U.S. News & World Report'' in 2022. It was the highest-ranking Russian educational ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zaitsev's Rule
In organic chemistry, Zaitsev's rule (or Saytzeff's rule, Saytzev's rule) is an empirical rule for predicting the favored alkene product(s) in elimination reactions. While at the University of Kazan, Russian chemist Alexander Zaitsev studied a variety of different elimination reactions and observed a general trend in the resulting alkenes. Based on this trend, Zaitsev proposed that the alkene formed in greatest amount is that which corresponded to removal of the hydrogen from the alpha-carbon having the fewest hydrogen substituents. For example, when 2-iodobutane is treated with alcoholic potassium hydroxide (KOH), 2-butene is the major product and 1-butene is the minor product. : More generally, Zaitsev's rule predicts that in an elimination reaction the most substituted product will be the most stable, and therefore the most favored. The rule makes no generalizations about the stereochemistry of the newly formed alkene, but only the regiochemistry of the elimination reaction. Wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reformatskii Reaction
The Reformatsky reaction (sometimes misspelled Reformatskii reaction) is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters: The organozinc reagent, also called a 'Reformatsky enolate', is prepared by treating an alpha-halo ester with zinc dust. Reformatsky enolates are less reactive than lithium enolates or Grignard reagents and hence nucleophilic addition to the ester group does not occur. The reaction was discovered by Sergey Nikolaevich Reformatsky. Some reviews have been published. In addition to aldehydes and ketones, it has also been shown that the Reformatsky enolate is able to react with acid chlorides, imines, nitriles (see Blaise reaction), and nitrones. Moreover, metals other than zinc have also been used, including magnesium, iron, cobalt, nickel, germanium, cadmium, indium, barium, and cerium. Additionally, metal salts are also applicable in place of metals, notably samarium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reaction
The Grignard reaction () is an organometallic chemical reaction in which alkyl, allyl, vinyl, or aryl-magnesium halides ( Grignard reagent) is added to a carbonyl group in an aldehyde or ketone. This reaction is important for the formation of carbon–carbon bonds. The reaction of an organic halide with magnesium is ''not'' a Grignard reaction, but provides a Grignard reagent. : Grignard reactions and reagents were discovered by and are named after the French chemist François Auguste Victor Grignard (University of Nancy, France), who published it in 1900 and was awarded the 1912 Nobel Prize in Chemistry for this work. Reaction mechanism Because carbon is more electronegative than magnesium, the carbon attached to magnesium functions as a nucleophile and attacks the electrophilic carbon atom that is present within the polar bond of a carbonyl group. The addition of the Grignard reagent to the carbonyl typically proceeds through a six-membered ring transition state. Based on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sergei Nikolaevich Reformatskii
Sergey Nikolaevich Reformatsky (russian: Серге́й Никола́евич Реформа́тский) (April 1, 1860 – July 28, 1934) was a Russian chemist. Life He was born as a son of a preacher in Borisoglebskoe, near Ivanovo. He studied at the University of Kazan under Alexander Mikhailovich Zaitsev until 1882. He went to Germany for further studies. He joined Victor Meyer at the University of Heidelberg and Wilhelm Ostwald at the University of Leipzig and finally getting his Ph.D in 1891. The following year he was appointed professor at the University of Kyiv where he stayed the rest of his life. Work In 1887 discovered the Reformatsky reaction, during which a zinc organic compound is the key component. The use of zinc in organic reactions was common at that time, but it was subsequently replaced by the more convenient magnesium. This was not possible for the reaction of α-chloro acids with ketones, because the magnesium based Grignard reagent A Gri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yegor Yegorovich Vagner
Yegor Yegorovich Wagner (Russian Егор Егорович Вагнер, sometimes Georg Wagner or Egor Vagner; 9 December 1849, in Kazan – 27 November 1903, in Warsaw) was a Russian organic chemist, famous for the discovery of the "Wagner reaction", named after him. Former representative of the Kazan School of Chemistry. Early life Yegor Yegorovich Wagner's grandfather was August Wagner, a pharmacist from East Prussia East Prussia ; german: Ostpreißen, label=Low Prussian; pl, Prusy Wschodnie; lt, Rytų Prūsija was a province of the Kingdom of Prussia from 1773 to 1829 and again from 1878 (with the Kingdom itself being part of the German Empire from 187 .... In search of happiness, young August went to distant Russia, to the city of Kazan, where he opened his own pharmacy. His affairs were going well, which was largely facilitated by high qualifications and personal charm. After several years of work, August became a wealthy man and married a local girl from a Ge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosgene
Phosgene is the organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. Phosgene is a valued and important industrial building block, especially for the production of precursors of polyurethanes and polycarbonate plastics. Phosgene is extremely poisonous and was used as a chemical weapon during World War I, where it was responsible for 85,000 deaths. It was a highly potent pulmonary irritant and quickly filled enemy trenches due to it being a heavy gas. It is classified as a Schedule 3 substance under the Chemical Weapons Convention. In addition to its industrial production, small amounts occur from the breakdown and the combustion of organochlorine compounds, such as chloroform. Structure and basic properties Phosgene is a planar molecule as predicted by VSEPR theory. The C=O distance is 1.18 Å, the C−Cl distance is 1.74 Å and the Cl−C−Cl angle is 111 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylzinc
Dimethylzinc, also known as Zinc methyl, DMZ, or DMZn is a colorless volatile liquid Zn(CH3)2, formed by the action of methyl iodide on zinc at elevated temperature or on zinc sodium alloy. :2Zn + 2CH3I → Zn(CH3)2 + ZnI2 The sodium assists the reaction of the zinc with the methyl iodide. Zinc iodide is formed as a byproduct. It has a disagreeable odor, and is pyrophoric. It has been of great importance in the synthesis of organic compounds. It is soluble in alkanes and often sold as a solution in hexanes. It belongs to the large series of similar compounds such as diethylzinc. History This substance was first prepared by Edward Frankland during his work with Robert Bunsen in 1849 at the University of Marburg. After heating a mixture of zinc and methyl iodide in an airtight vessel, a flame burst out when the seal was broken. In the laboratory, this synthesis method remains unchanged today, except that copper or copper compounds are used to activate the zinc. Uses Dimethyl zi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |