|
Aggresome
In eukaryotic cells, an aggresome refers to an aggregation of misfolded proteins in the cell, formed when the protein degradation system of the cell is overwhelmed. Aggresome formation is a highly regulated process that possibly serves to organize misfolded proteins into a single location. Biogenesis Correct folding requires proteins to assume one particular structure from a constellation of possible but incorrect conformations. The failure of polypeptides to adopt their proper structure is a major threat to cell function and viability. Consequently, elaborate systems have evolved to protect cells from the deleterious effects of misfolded proteins. Cells mainly deploy three mechanisms to counteract misfolded proteins: up-regulating chaperones to assist protein refolding, proteolytic degradation of the misfolded/damaged proteins involving ubiquitin–proteasome and autophagy–lysosome systems, and formation of detergent-insoluble aggresomes by transporting the misfolded protei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewy Bodies
Lewy bodies are the inclusion bodies – abnormal aggregations of protein – that develop inside nerve cells affected by Parkinson's disease (PD), the Lewy body dementias ( Parkinson's disease dementia and dementia with Lewy bodies (DLB)), and some other disorders. They are also seen in cases of multiple system atrophy, particularly the parkinsonian variant (MSA-P). Lewy bodies appear as spherical masses in the cytoplasm that displace other cell components. For instance, some Lewy bodies tend to displace the nucleus to one side of the cell. There are two main kinds of Lewy bodies: classical and cortical. Lewy bodies may be found in the midbrain (within the substantia nigra) or within the cortex. A classical Lewy body is an eosinophilic cytoplasmic inclusion consisting of a dense core surrounded by a halo of 10-nm-wide radiating fibrils, the primary structural component of which is alpha-synuclein. History In 1910, Fritz Heinrich Lewy was studying in Berlin for his doctora ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Misfolded Proteins
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproducible process, a polypeptide folds into its characteristic three-dimensional structure from a random coil. Each protein exists first as an unfolded polypeptide or random coil after being translated from a sequence of mRNA to a linear chain of amino acids. At this stage the polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). As the polypeptide chain is being synthesized by a ribosome, the linear chain begins to fold into its three-dimensional structure. Folding of many proteins begins even during translation of the polypeptide chain. Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), k ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytotoxic
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa''). Cell physiology Treating cells with the cytotoxic compound can result in a variety of cell fates. The cells may undergo necrosis, in which they lose membrane integrity and die rapidly as a result of cell lysis. The cells can stop actively growing and dividing (a decrease in cell viability), or the cells can activate a genetic program of controlled cell death (apoptosis). Cells undergoing necrosis typically exhibit rapid swelling, lose membrane integrity, shut down metabolism, and release their contents into the environment. Cells that undergo rapid necrosis in vitro do not have sufficient time or energy to activate apoptotic machinery and will not express apoptotic markers. Apoptosis is characterized by well defined cytological and molecular events including a cha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurodegenerative
A neurodegenerative disease is caused by the progressive loss of structure or function of neurons, in the process known as neurodegeneration. Such neuronal damage may ultimately involve cell death. Neurodegenerative diseases include amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease, Alzheimer's disease, Huntington's disease, multiple system atrophy, and prion diseases. Neurodegeneration can be found in the brain at many different levels of neuronal circuitry, ranging from molecular to systemic. Because there is no known way to reverse the progressive degeneration of neurons, these diseases are considered to be incurable; however research has shown that the two major contributing factors to neurodegeneration are oxidative stress and inflammation. Biomedical research has revealed many similarities between these diseases at the subcellular level, including atypical protein assemblies (like proteinopathy) and induced cell death. These similarities suggest that t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parkinson's Disease
Parkinson's disease (PD), or simply Parkinson's, is a long-term degenerative disorder of the central nervous system that mainly affects the motor system. The symptoms usually emerge slowly, and as the disease worsens, non-motor symptoms become more common. The most obvious early symptoms are tremor, rigidity, slowness of movement, and difficulty with walking. Cognitive and behavioral problems may also occur with depression, anxiety, and apathy occurring in many people with PD. Parkinson's disease dementia becomes common in the advanced stages of the disease. Those with Parkinson's can also have problems with their sleep and sensory systems. The motor symptoms of the disease result from the death of cells in the substantia nigra, a region of the midbrain, leading to a dopamine deficit. The cause of this cell death is poorly understood, but involves the build-up of misfolded proteins into Lewy bodies in the neurons. Collectively, the main motor symptoms are also k ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-synuclein
Alpha-synuclein is a protein that, in humans, is encoded by the ''SNCA'' gene. Alpha-synuclein is a neuronal protein that regulates synaptic vesicle trafficking and subsequent neurotransmitter release. It is abundant in the brain, while smaller amounts are found in the heart, muscle and other tissues. In the brain, alpha-synuclein is found mainly in the axon terminals of presynaptic neurons. Within these terminals, alpha-synuclein interacts with phospholipids and proteins. Presynaptic terminals release chemical messengers, called neurotransmitters, from compartments known as synaptic vesicles. The release of neurotransmitters relays signals between neurons and is critical for normal brain function. The human alpha-synuclein protein is made of 140 amino acids. An alpha-synuclein fragment, known as the non- Abeta component (NAC) of Alzheimer's disease amyloid, originally found in an amyloid-enriched fraction, was shown to be a fragment of its precursor protein, NACP. It was later de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
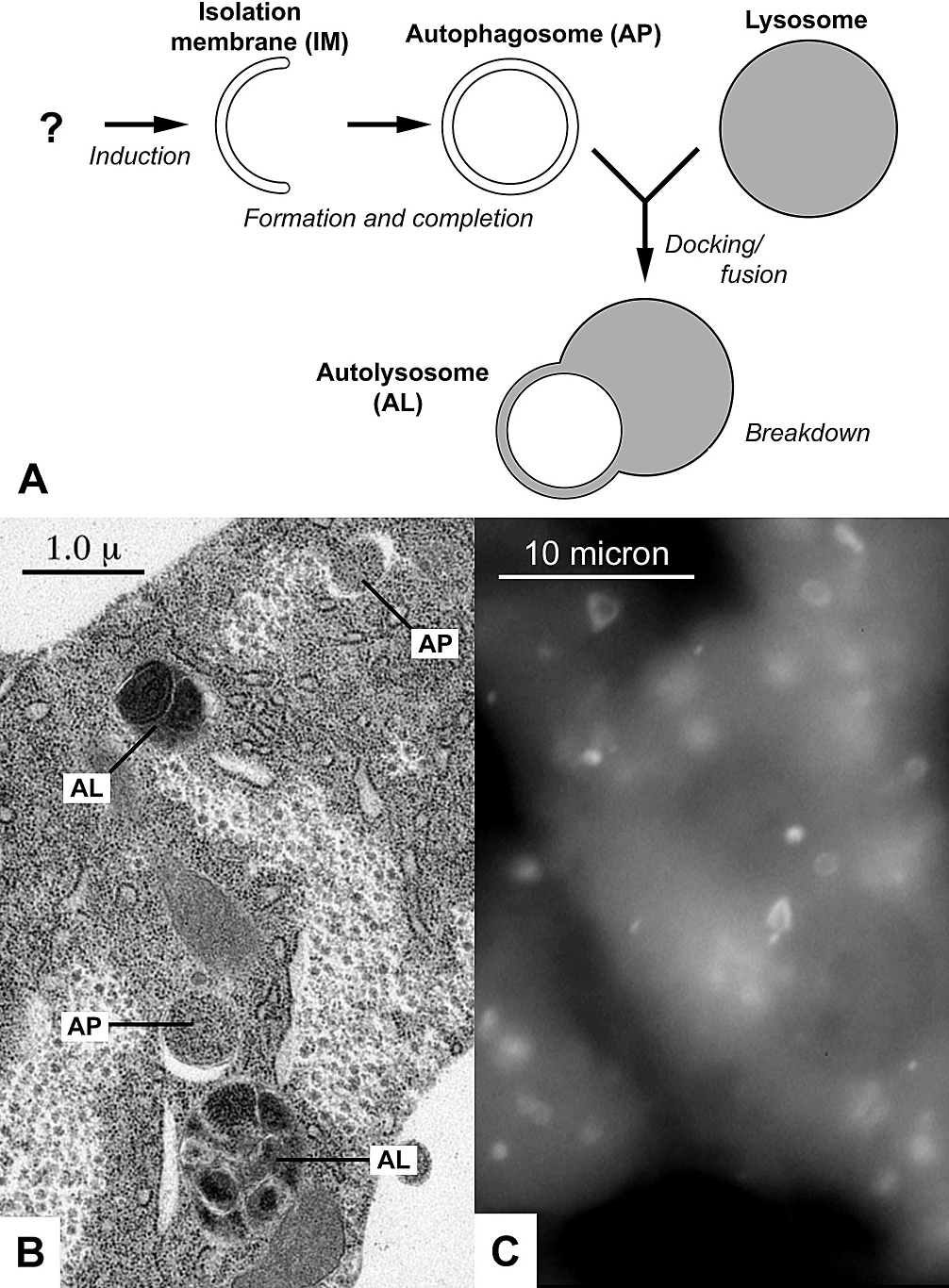
Autophagic
Autophagy (or autophagocytosis; from the Ancient Greek , , meaning "self-devouring" and , , meaning "hollow") is the natural, conserved degradation of the cell that removes unnecessary or dysfunctional components through a lysosome-dependent regulated mechanism. It allows the orderly degradation and recycling of cellular components. Although initially characterized as a primordial degradation pathway induced to protect against starvation, it has become increasingly clear that autophagy also plays a major role in the homeostasis of non-starved cells. Defects in autophagy have been linked to various human diseases, including neurodegeneration and cancer, and interest in modulating autophagy as a potential treatment for these diseases has grown rapidly. Four forms of autophagy have been identified: macroautophagy, microautophagy, chaperone-mediated autophagy (CMA), and crinophagy. In macroautophagy (the most thoroughly researched form of autophagy), cytoplasmic components (like mit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mediator (coactivator)
Mediator is a multiprotein complex that functions as a transcriptional coactivator in all eukaryotes. It was discovered in 1990 in the lab of Roger D. Kornberg, recipient of the 2006 Nobel Prize in Chemistry. Mediator complexes interact with transcription factors and RNA polymerase II. The main function of mediator complexes is to transmit signals from the transcription factors to the polymerase. Mediator complexes are variable at the evolutionary, compositional and conformational levels. The first image shows only one "snapshot" of what a particular mediator complex might be composed of, but it certainly does not accurately depict the conformation of the complex ''in vivo''. During evolution, mediator has become more complex. The yeast ''Saccharomyces cerevisiae'' (a simple eukaryote) is thought to have up to 21 subunits in the core mediator (exclusive of the CDK module), while mammals have up to 26. Individual subunits can be absent or replaced by other subunits under differe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HSP70
The 70 kilodalton heat shock proteins (Hsp70s or DnaK) are a family of conserved ubiquitously expressed heat shock proteins. Proteins with similar structure exist in virtually all living organisms. Intracellularly localized Hsp70s are an important part of the cell's machinery for protein folding, performing chaperoning functions, and helping to protect cells from the adverse effects of physiological stresses. Additionally, membrane-bound Hsp70s have been identified as a potential target for cancer therapies and their extracellularly localized counterparts have been identified as having both membrane-bound and membrane-free structures. Discovery Members of the Hsp70 family are very strongly upregulated by heat stress and toxic chemicals, particularly heavy metals such as arsenic, cadmium, copper, mercury, etc. Heat shock was originally discovered by Ferruccio Ritossa in the 1960s when a lab worker accidentally boosted the incubation temperature of Drosophila (fruit flies). Wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BAG3
BAG family molecular chaperone regulator 3 is a protein that in humans is encoded by the ''BAG3'' gene. BAG3 is involved in chaperone-assisted selective autophagy. Function BAG proteins compete with Hip-1 for binding to the Hsc70/Hsp70 ATPase domain and promote substrate release. All the BAG proteins have an approximately 45-amino acid BAG domain near the C terminus but differ markedly in their N-terminal regions. The protein encoded by this gene contains a WW domain in the N-terminal region and a BAG domain in the C-terminal region. The BAG domains of BAG1, BAG2, and BAG3 interact specifically with the Hsc70 ATPase domain ''in vitro'' and in mammalian cells. All 3 proteins bind with high affinity to the ATPase domain of Hsc70 and inhibit its chaperone activity in a Hip-repressible manner. Clinical significance BAG gene has been implicated in age related neurodegenerative diseases such as Alzheimer's. It has been demonstrated that BAG1 and BAG3 regulate the proteasomal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
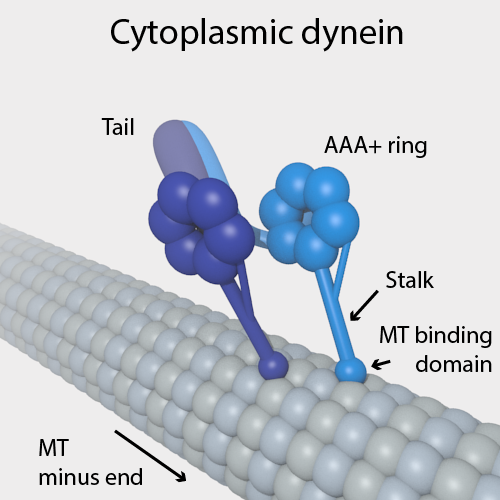
Dynein
Dyneins are a family of cytoskeletal motor proteins that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella. All of these functions rely on dynein's ability to move towards the minus-end of the microtubules, known as retrograde transport; thus, they are called "minus-end directed motors". In contrast, most kinesin motor proteins move toward the microtubules' plus-end, in what is called anterograde transport. Classification Dyneins can be divided into two groups: cytoplasmic dyneins and axonemal dyneins, which are also called ciliary or flagellar dyneins. * cytoplasmic ** heavy chain: DYNC1H1, DYNC2H1 ** intermediate chain: DYNC1I1, DYNC1I2 ** light intermediate chain: DYNC1LI1, DYNC1LI2, DYNC2LI1 ** light chain: DYNLL1, DYNLL2, DYNLRB1, DYNLRB2, DYNLT1, DYNLT3 * axo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyubiquitination
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Four genes in the human genome code for ubiquitin: UBB, UBC, UBA52 and RPS27A. The addition of ubiquitin to a substrate protein is called ubiquitylation (or, alternatively, ubiquitination or ubiquitinylation). Ubiquitylation affects proteins in many ways: it can mark them for degradation via the proteasome, alter their cellular location, affect their activity, and promote or prevent protein interactions. Ubiquitylation involves three main steps: activation, conjugation, and ligation, performed by ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s), respectively. The result of this sequential cascade is to bind ubiquitin to lysine residues on the protein substrate via an isopeptide bond, cy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |