|
Affinine
Affinine is a monoterpenoid indole alkaloid which can be isolated from plants of the genus '' Tabernaemontana''. Structurally it can be considered a member of the vobasine alkaloid family and may be synthesized from tryptophan. Limited pharmacological testing has indicated that it may be an effective inhibitor of both acetylcholinesterase Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme Enzymes () are proteins that a ... and butyrylcholinesterase. See also * Affinisine References Acetylcholinesterase inhibitors Alkaloids found in Apocynaceae {{organic-chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affinisine
Affinisine is a monoterpenoid indole alkaloid which can be isolated from plants of the genus ''Tabernaemontana''. Structurally, it can be considered a member of the sarpagine alkaloid family and may be synthesized from tryptophan via a Pictet-Spengler reaction. Pharmacology Limited pharmacological testing has indicated that affinisine may effectively inhibit acetylcholinesterase and butyrylcholinesterase. See also * Affinine Affinine is a monoterpenoid indole alkaloid which can be isolated from plants of the genus ''Tabernaemontana''. Structurally it can be considered a member of the vobasine alkaloid family and may be synthesized from tryptophan. Limited pharmacolog ... * 19-Epivoacristine References Acetylcholinesterase inhibitors Quinolizidine alkaloids Indoles {{alkaloid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tabernaemontana
''Tabernaemontana'' is a genus of flowering plants in the family Apocynaceae. It has a pan-tropical distribution, found in Asia, Africa, Australia, North America, South America, and a wide assortment of oceanic islands. These plants are evergreen shrubs and small trees growing to 1–15 m tall. The leaves are opposite, 3–25 cm long, with milky sap; hence it is one of the diverse plant genera commonly called "milkwood". The flowers are fragrant, white, 1–5 cm in diameter. The cultivar '' T. divaricata'' cv. 'Plena', with doubled-petaled flowers, is a popular houseplant. Some members of the genus ''Tabernaemontana'' are used as additives to some versions of the psychedelic drink ayahuasca; the genus is known to contain ibogaine (e.g. in bëcchëte, ''T. undulata''), conolidine (present in minor concentration in ''T. divaricata'') and voacangine ('' T. alba'', '' T. arborea'', '' T. africana''). Because of presence of coronaridine and voacangine in Mexican ''Tabernae ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoterpenoid
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries. Biosynthesis Monoterpenes are derived biosynthetically from units of isopentenyl pyrophosphate, which is formed from acetyl-CoA via the intermediacy of mevalonic acid in the HMG-CoA reductase pathway. An alternative, unrelated biosynthesis pathway of IPP is known in some bacterial groups and the plastids of plants, the so-called MEP-(2-methyl-D-erythritol-4-phosphate) pathway, which is initiated from Pentose, C5 sugars. In both pathways, IPP is isomerized to DMAPP by the enzyme isopentenyl pyrophosphate isomerase. Geranyl pyrophosphate is the pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.Chemical Encyclopedia: alkaloids xumuk.ru Alkaloids are produced by a large variety of organisms including , , Medicinal plant, plants, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vobasine
Vobasine is a naturally occurring monoterpene indole alkaloid found in several species in the genus ''Tabernaemontana'' including ''Tabernaemontana divaricata''. History Vobasine was first reported by Renner in 1959 after its isolation from ''Voacanga africana''. The two structurally related compounds, dregamine and tabernaemontanine, where its alkene (=CHCH3) sidechain was reduced to ethyl groups in two configurations, had their relationship confirmed in the 1970s. Vobasine has been found in many plants of the dogbane (Apocynaceae) family including '' Tabernaemontana dichotoma''. Synthesis Biosynthesis As with other Indole alkaloids, the biosynthesis of vobasine starts from the amino acid tryptophan. This is converted into strictosidine before further elaboration. Chemical synthesis The synthesis of alkaloids with the same carbon skeleton as vobasine began in the 1960s and has continued, with some work providing enantiospecific approaches to closely-related compounds. Natur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptophan
Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α- carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. It is essential in humans, meaning that the body cannot synthesize it and it must be obtained from the diet. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3. It is encoded by the codon UGG. Like other amino acids, tryptophan is a zwitterion at physiological pH where the amino group is protonated (–; pKa = 9.39) and the carboxylic acid is deprotonated ( –COO−; pKa = 2.38). Humans and many animals cannot synthesize tryptophan: they need to obtain it through their diet, making it an essential amino acid. Function Amino acids, including tryptophan, are used as building blocks in protein biosynthesis, and proteins are required to sustain life. Man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
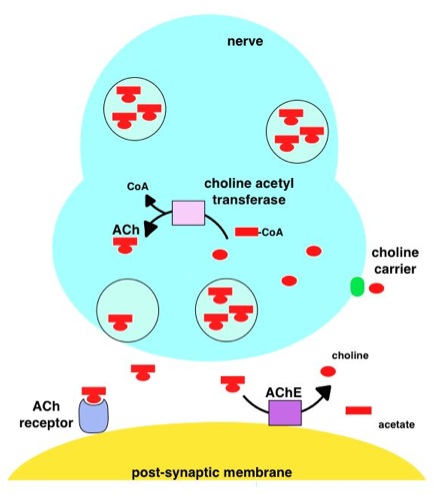
Acetylcholinesterase
Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ... that catalysis, catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters: : acetylcholine + H2O = choline + acetate It is found at mainly neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate neurotransmission, synaptic transmission. It belongs to the carboxylesterase family of enzymes. It is the primary target of inhibition by organophosphorus compounds such as nerve agents and pesticides. Enzyme structure and mechani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyrylcholinesterase
Butyrylcholinesterase (HGNC symbol BCHE; EC 3.1.1.8), also known as BChE, BuChE, BuChase, pseudocholinesterase, or plasma (cholin)esterase, is a nonspecific cholinesterase enzyme that hydrolyses many different choline-based esters. In humans, it is made in the liver, found mainly in blood plasma, and encoded by the ''BCHE'' gene. It is very similar to the neuronal acetylcholinesterase, which is also known as RBC or erythrocyte cholinesterase. The term "serum cholinesterase" is generally used in reference to a clinical test that reflects levels of both of these enzymes in the blood. Assay of butyrylcholinesterase activity in plasma can be used as a liver function test as both hypercholinesterasemia and hypocholinesterasemia indicate pathological processes. The half-life of BCHE is approximately 10 to 14 days. Butyrylcholine is a synthetic compound that does not occur in the body naturally. It is used as a tool to distinguish between acetylcholinesterase and butyrylcholinesterase. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylcholinesterase Inhibitors
Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level and duration of action of acetylcholine in the central nervous system, autonomic ganglia and neuromuscular junctions, which are rich in acetylcholine receptors. Acetylcholinesterase inhibitors are one of two types of cholinesterase inhibitors; the other being butyryl-cholinesterase inhibitors. Acetylcholinesterase is the primary member of the cholinesterase enzyme family. Acetylcholinesterase inhibitors are classified as reversible, irreversible, or quasi-irreversible (also called pseudo-irreversible). Mechanism of action Organophosphates Organophosphates like TEPP and sarin inhibit cholinesterases, enzymes that hydrolyze the neurotransmitter acetylcholine. The active centre of cholinesterases feature two important sites, namely th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |