|
Acidification (chemistry) , a cooking technique
{{disambiguation ...
Acidification may refer to: * Ocean acidification, decrease in the pH of the Earth's oceans * Freshwater acidification, atmospheric depositions and soil leaching of SOx and NOx * Soil acidification, buildup of hydrogen cations, which reduces the soil pH * Souring Souring is a food preparation technique that causes a physical and chemical change in food by exposing it to an acid. This acid can be added explicitly (e.g., vinegar, lemon juice, lime juice, etc.), or can be produced within the food itself by a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ocean Acidification
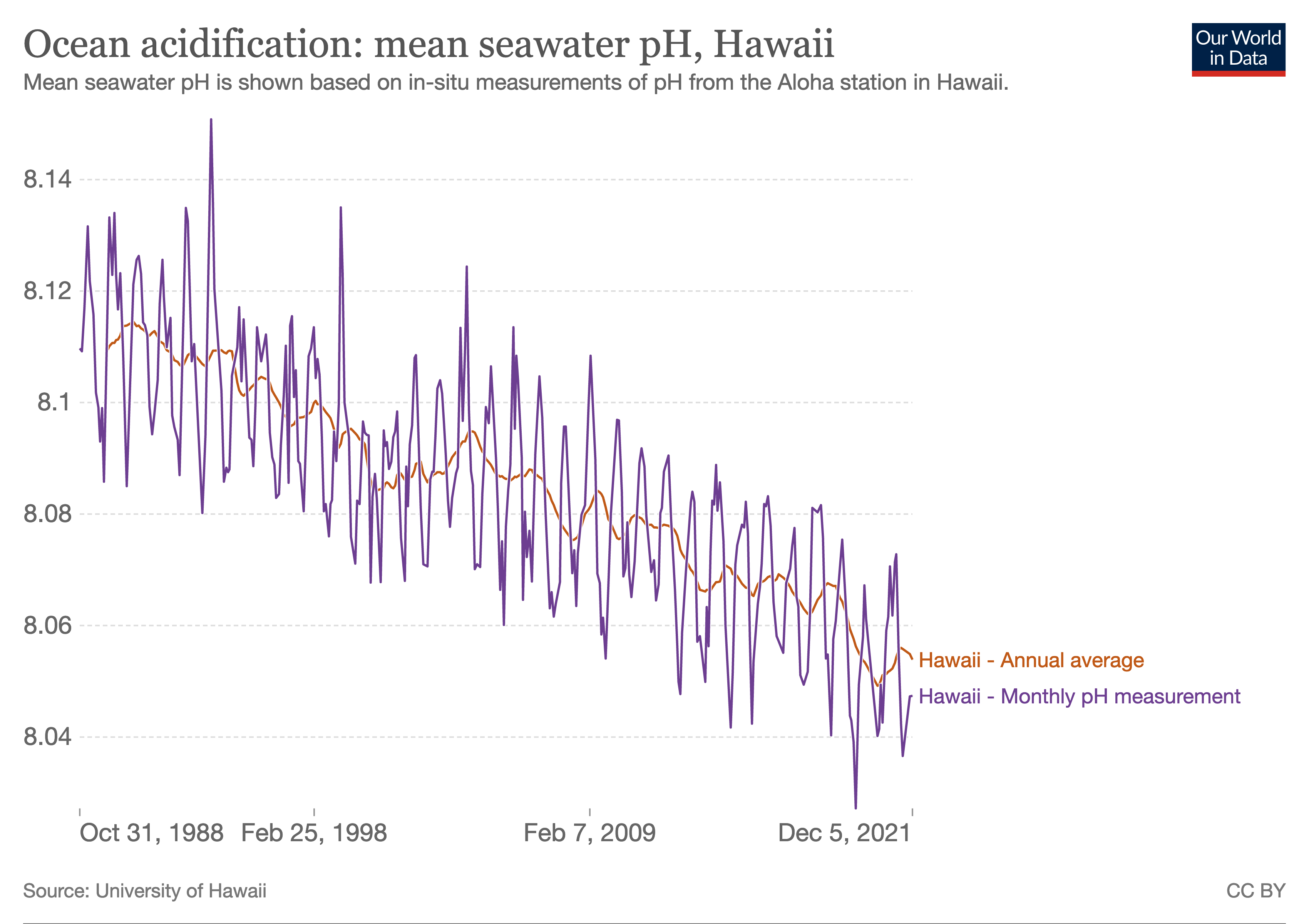
Ocean acidification is the reduction in the pH value of the Earth’s ocean. Between 1751 and 2021, the average pH value of the ocean surface has decreased from approximately 8.25 to 8.14. The root cause of ocean acidification is carbon dioxide emissions from human activities which have led to atmospheric carbon dioxide (CO2) levels of more than 410 ppm (in 2020). The oceans absorb CO2 from the atmosphere. This leads to the formation of carbonic acid (H2CO3) which dissociates into a bicarbonate ion () and a hydrogen ion (H+). The free hydrogen ions (H+) decrease the pH of the ocean, therefore increasing acidity (this does not mean that seawater is acidic yet; it is still alkaline, with a pH higher than 8). A decrease in pH corresponds to a decrease in the concentration of carbonate ions, which are the main building block for calcium carbonate (CaCO3) shells and skeletons. Marine calcifying organisms, like mollusks, oysters and corals, are particularly affected by this as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Freshwater Acidification
Freshwater acidification occurs when acidic inputs enter a body of fresh water through the weathering of rocks, invasion of acidifying gas (e.g. carbon dioxide), or by the reduction of acid anions, like sulfate and nitrate within the lake. Freshwater acidification is primarily caused by sulfur oxides (SOx) and nitrogen oxides (NOx) entering the water from atmospheric depositions and soil leaching. Carbonic acid and dissolved carbon dioxide can also enter freshwaters in a similar manner associated with runoff through carbon dioxide-rich soils. Runoff that contains these compounds may be accompanied by acidifying hydrogen ions and inorganic aluminum, which can be toxic to marine organisms. Acid rain is also a contributor to freshwater acidification. It is created when SOx and NOx react with water, oxygen, and other oxidants within the clouds. The buffering capacity of soils and bedrocks within the freshwater ecosystem can contribute to the acidity of the water. Each freshwater reserv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soil Acidification
Soil acidification is the buildup of hydrogen cations, which reduces the soil pH. Chemically, this happens when a proton donor gets added to the soil. The donor can be an acid, such as nitric acid, sulfuric acid, or carbonic acid. It can also be a compound such as aluminium sulfate, which reacts in the soil to release protons. Acidification also occurs when base cations such as calcium, magnesium, potassium and sodium are leached from the soil. Soil acidification naturally occurs as lichens and algae begin to break down rock surfaces. Acids continue with this dissolution as soil develops. With time and weathering, soils become more acidic in natural ecosystems. Soil acidification rates can vary, and increase with certain factors such as acid rain, agriculture, and pollution. Causes Acid rain Rainfall is naturally acidic due to carbonic acid forming from carbon dioxide in the atmosphere. This compound causes rainfall pH to be around 5.0-5.5. When rainfall has a lower pH than na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |